References
Δt week
n (P/O)
Dose (mg/day)
Δweight (P)
Δweight (SA)
Comments
[55]
12
19/20
150
−2.1 kg
−4.3 kg
First clinical study
[56]
12
39/37
30
−3.2 kg
−3.6 kg
Different dosages
39/45
180
−3.2 kg
−3.9 kg
39/47
360
−3.2 kg
−4.8 kg
Δweight SS p < 0.01
[57]
24
136/134
90
−6.5 %
−8.5 %
NS; different dosages
136/135
120
−6.5 %
−8.8 %
Δweight SS p < 0.002
136/136
360
−6.5 %
−9.8 %
Δweight SS p < 0.002
136/135
720
−6.5 %
−9.3 %
Δweight SS p < 0.002
[58]
52
23/23
360
−2.6 %
−8.4 %
Δweight SS p < 0.001
[59]
52
113/115
360
−5.4 %
−8.5 %
[60]
52
186/1 O
360
−4.6 %
−5.9 %
Coronary risk
[52]
104
343/345
360
−6.1 %
−10.2 %
Δweight after 1 year
[50]
104
223/657
360
−4.5 %
−7.6 %
Δweight SS p < 0.001
[61]
104
265/266
180
−4.1 kg
−7.1 kg
Δweight after 1 year
265/264
360
−4.1 kg
−7.0 kg
[64]
104
243/242
180
−6.6 %
−7.6 %
Δweight after 1 year
243/244
360
−6.6 %
−9.7 %
[62]
104
316/35
360
−3.8 kg
−6.7 kg
IGT progression
[63]
104
36/36
360
−8.6 kg
−13.1 kg
[53]
52
159/162
360
−4.3 %
−6.2 %
Diabetics SS p < 0.001
[54]
24
174/164
360
−3 %
−4.7 %
Diabetics SS p < 0.001
The main adverse effects of orlistat are gastrointestinal in nature and include diarrhea, flatulence and fecal urgency and incontinence. In May 2010, the FDA issued a drug safety communication about a possible relationship between the product and isolated cases of severe hepatic insufficiency (13 cases), with three hepatic transplants and two deaths. There is no pharmacokinetic explanation for these cases. Another adverse effect recently published has been acute kidney injury (AKI) induced by oxalate. A 2 % increase in AKI was demonstrated in a revision of 953 patients [33]. The malabsorbed fat is able to bind to enteric calcium, increasing free oxalate absorption and leading to hyperoxaluria, a risk factor for kidney stones. The poor fat absorption is also likely responsible for serum level reductions of liposoluble vitamins (A, D, E, and K) and could reduce the absorption of some medications.
Orlistat 120 mg is given three times a day, at breakfast, lunch, and dinner. In Brazil and other countries in which breakfast is much smaller than lunch and dinner, a twice daily dose is acceptable. With our experience in the management of obesity, we consider orlistat a good choice of treatment for hyperphagic patients (who eat proportionally more total calories and fat in meals than in snacks); for patients on weight maintenance regimens after weight loss; and for individuals with high LDL, NASH or a high risk of diabetes. We also consider the drug in combination (see below).
Sibutramine
Sibutramine is the most studied antiobesity drug, but it was withdrawn from the market of the vast majority of countries due to the SCOUT Study, although still available in Brazil upon special medical order [26].
It was obtained after modifications in the chemical structure of amphetamine, being considered a beta-phenethylamine derivative. Sibutramine acts by blocking norepinephrine and serotonin uptake in the synapsis, leading to a reduction in food intake by increasing satiety. An increased thermogenesis was also demonstrated in mice [34].
In a meta-analysis, the placebo subtracted weight loss was 4.2 kg (4.3 %) with sibutramine. In addition, sibutramine treatment increased the absolute percentage of 5 % and 10 % responders by 32 % (55 % vs. 27 %) and 18 % (28 % vs. 10 %), respectively [29].
Treatment with sibutramine significantly reduces waist circumference and triglyceride concentration and increases concentrations of HDL-cholesterol. LDL-cholesterol was not significantly reduced and glycemic indexes only reduced in those who lost more weight [35].
The main adverse effect is an increase in sympathetic tone, which leads to a small increase in pulse and blood pressure. Due to this effect, the drug was always contraindicated in subjects with established cardiovascular disease.
The SCOUT Study (Sibutramine Cardiovascular OUTcomes Trial) was designed to assess the cardiovascular safety of the drug and was conducted in a high-risk population (diabetic patients with one or more risk factors for CVD, previous history of CVD and diabetic with established CVD) [26]. A discrete, but significant, increase in nonfatal cardiovascular events was observed in the intervention group (11.4 %) compared to placebo (10 %). This 16 % increases lead the EMEA (European Medicine Agency) to withdraw sibutramine from the European Market. Afterwards, Abbott (who have the commercialization rights of the drug in the USA) voluntarily withdrew the drug from the North-American market before an official statement from the FDA, but the drug never achieved popularity in that country. In Brazil, the drug still is sold, but with severe restrictions for its prescription and acquisition made by ANVISA, Brazil’s local regulatory agency.
A sub-analysis of the SCOUT report recently suggested that modest weight loss over short-term and long-term periods was associated with reductions in subsequent cardiovascular mortality for the following 4–5 years, even in those with preexisting cardiovascular disease [36]. The increased nonfatal cardiovascular events observed in the overall analysis probably represent individuals who did not achieve weight loss, but persisted in the intervention group due to the study design, something that does not happen in clinical practice.
The drug should not be used in combination with other serotonin uptake inhibitors, like many antidepressants, due to a risk of serotoninergic syndrome, a very rare but potentially severe condition.
Catecholaminergic Agents: Phentermine and Diethylpropion
Phentermine is an amphetamine analogue which promotes weight loss, acting as an appetite suppressant that interacts with amine transporters in the central nervous system (Fig. 41.1) [37]. Its main action is in the norepinephrine transporter, leading to a greater release of norepinephrine in the synapsis. It also has a weak activity in the dopamine transporter and an even weaker one in the serotonin transporter. The drug has been approved for the short-term treatment of obesity since 1959 and is the most widely prescribed antiobesity medication in the USA, and was even prior the withdrawal of sibutramine. As it was approved long before the modern rules and regulations for drug marketing and is a very cheap medication with no patent owner, long-term clinical trials were never conducted. A meta-analysis of its safety, analyzing six randomized trials, showed a modest placebo-subtracted weight loss of 3.6 kg, with large heterogeneity between the studies, with lengths ranging from 2 to 24 weeks [38]. The main adverse effects are anxiety, insomnia, and sympathetic activation (a rise in blood pressure and tachycardia) and the drug is contraindicated in people with underlying cardiovascular disease and severe psychiatric conditions. Contrary to what many physicians believe, due to the chemical resemblance with amphetamine there is limited dependence liability with this medication (FDA DEA schedule IV). In current clinical practice in the USA, any use of phentermine for longer than 12 weeks is considered off-label and the drug is unavailable in several parts of the world, such as in Brazil and Europe.
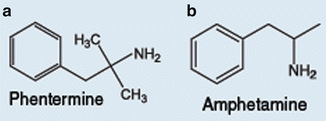

Fig. 41.1
Molecular structure of phentermine and amphetamine
Recently, the combination of phentermine with the antiepileptic drug topiramate has been approved in the USA for long-term obesity treatment (see below).
Diethylpropion has a similar mechanism of action, as well as indications and contraindications, to phentermine [39]. It is also poorly studied and less prescribed than phentermine.
Lorcaserin
Lorcaserin is a selective serotonin receptor (5-HT2C) agonist with a much greater 5-HT2c selectivity, than 5-HT2b [40]. This specificity appears to be important in reducing the risk of valvulopathy detected with similar older compounds such as phenfluramine, withdrawn from the market in 1997 (used in a fixed dose combination with phentermine) after 24 reports of valvular dysfunctions in individuals on continued use of this drug, as cardiac valves have mainly the 5-HT2b subtype [41].
The serotonergic stimulus activates the POMC system and diminishes caloric intake while increasing catabolism via second-order effectors, such as TRH, CRH and MC4R [42]. Some human studies have detected an increased basal metabolic rate and thermogenesis, but this result was not reproducible in all experiments.
The most studied dose was 10 mg bid [40]. A phase three study, BLOOM, randomized 3,182 obese subjects (BMI 30–45 kg/m2 or 27–30 kg/m2 with obesity-related comorbidities). The placebo-subtracted weight loss was 3.6 % (5.8 % vs. 2.2 %) and the 5 % weight loss was 47 % compared with 20 % in placebo. More patients also lost 10 % or more of their baseline body weight in the lorcaserin group than in the placebo group (22.6 % vs. 7.7 %) [40].
The rate of cardiac valvulopathy was not increased with the use of lorcaserin after serial echocardiography analysis. The most frequent adverse events reported were headache, dizziness, and nausea, with similar rates of serious adverse events compared to placebo. The FDA approved lorcaserin after more than 13 years since its last antiobesity drug approval.
Although the results were only reasonable, we think lorcaserin could be a good option for patients who cannot use or tolerate other drugs.
New Drugs
There are several research projects on the development of new drugs for obesity treatment. To date, tesofensine, a serotonin–norepinephrine reuptake inhibitor and cetilistat, a drug with similar mechanisms than orlistat, are the most advanced, in phase two studies [32, 43]. See Table 41.2.
Table 41.2
New perspectives for obesity treatment
Drugs | Studies | N | Weeks | Placebo subtracted weight loss (%) |
|---|---|---|---|---|
Lorcaserin (FDA-approved) | BLOOM (phase 3) | 3,182 | 52 | 3.6 |
BLOSSOM (phase 3) | 4,008 | 52 | 3.1 | |
Phentermine + Topiramate (FDA-approved) | EQUIP (phase 3) | 1,267 | 56 | 9.4 (higher dose) |
CONQUER (phase 3) | 2,487 | 56 | 8.6 (higher dose) | |
Tesofensin | TIPO-1 (phase 2) | 203 | 24 | 9.2 (0.5 mg) |
Liraglutide | Phase 2 | 564 | 20 | 4.5 (3.0 mg) |
Cetilistat | Phase 2 | 612 | 12 | 1.3 (120 mg) |
Bupropion + Naltrexone | COR-I (phase 3) | 1,742 | 60 | 4.8 |
COR-II (phase 3) | 1,496 | 60 | 5.2 | |
Bupropion + Zonisamide | (Phase 2) | 320 | 24 | 6.1 |
Pramlintide + Metreleptin | (Phase 2) | 139 | 24 | – |
Off-Label Drugs
As cited before, a substantial number of obesity specialists in the USA (and around the world) admitted to prescribing off label medications for weight loss [27]. We will briefly describe the most commonly used drugs for the treatment of obesity that are not officially approved for such use.
Topiramate
Topiramate, a monosaccharide d-fructose derivative, is an antiepileptic and migraine prophylaxis drug that commonly results in weight loss [44]. Its mechanisms of action are not fully understood and are very complex, involving many pathways. It enhances GABAergic activity via chloride channels, antagonizes NMDA-glutamate receptors, blocks voltage-dependent sodium channels, and also weakly antagonizes carbonic anhydrase isoenzyme subtypes II and IV [44]. The exact mechanisms by which all these actions promote weight loss are speculative, but clinically it has been shown to have a great impact in reducing binge-eating episodes and cravings, eating behaviors which affect a substantial percentage of obese individuals [45]. More recently, it has been proposed that topiramate exerts its effects on weight by improving hypothalamic leptin and insulin signaling and action [46]. The same study, on rodents receiving topiramate, found an increase in anorexigenic peptides and improved energy metabolism in peripheral tissues due to AMPK-signaling.
A large number of placebo-randomized trials have evaluated the efficacy and safety of topiramate as a long-term weight-loss drug. A recent meta-analysis of ten randomized trials confirmed a significant placebo-subtracted weight loss of 5.34 kg and a sixfold increased chance of significant weight loss (5 % or 10 %), although dosages varied considerably [47]. The largest study conducted over 60 weeks used dosages of 96, 192, and 256 mg, with mean percentage weight losses of 7.1 %, 9.1 %, and 9.7 %, respectively (versus 1.7 % in the placebo group) [48]. Other advantages of topiramate include the lack of a weight plateau during the first 6-months of treatment and a favorable metabolic profile, with reductions in insulin resistance, glucose intolerance and blood pressure [47], with positive results also observed in the type 2 diabetic populatio. The drug was also tested in obese patients with binge-eating disorders, with a reduction of bingeing scores and weight loss [45]. The main disadvantage of the drug is its adverse effect profile that leads to significant discontinuation rates, mainly due to paresthesias, memory/concentration impairment, and somnolence [48]. Nevertheless, severe adverse effects are rare and include nephrolithiasis, due to its carbonic anhydrase inhibition activity. This drug was never submitted for approval for obesity monotherapy, but has recently been used in a fixed-dose combination with phentermine (see below).
GLP-1 Analogs (Liraglutide and Exenatide)
GLP-1 analogs are antidiabetic drugs which frequently result in weight loss [49]. Due to this effect, these drugs are considered an excellent option for obese type 2 diabetic individuals. In the obese nondiabetic population, these drugs have also been studied in phase 2 studies, mainly liraglutide, with fairly good results, but have not yet been approved [50].
Liraglutide has 97 % structural homology with native GLP-1, but with a significantly larger half-life (13 h vs. 2 min) which allows a once daily regimen [49]. It leads to weight loss due to GLP-1 action in the gastrointestinal tract, reducing gastric emptying and promoting gastric distention which together increases satiety and satiation, but also acts on the CNS through its specific receptors, with not fully recognized mechanisms involving reduced energy intake and reduces the hedonic drive to eat [49].
The dosages used in phase 2 randomized-controlled trials for obesity are bigger than those for diabetes (up to 3.0 mg daily) [50]. After 1 year, the 3.0 mg dosage was associated with a placebo-subtracted weight loss of 4.5 % (7.2 kg vs. 2.8 kg) and 5 % and 10 % weight loss of 61 % vs. 29 %. The drug was also compared with orlistat and achieved better results (3.1 kg more weight loss and 32 % more 5 % weight-loss responders). Although 3.0 mg is not approved, doses of 1.8 mg were also superior to orlistat and the 1.2 mg dosage was similar. After 2 years, the results were sustained and the prevalence of prediabetes and metabolic syndrome decreased by 58 % in the 3.0 mg dose, with improvements in blood pressure and lipids. The biggest side effects were nausea and vomiting, mostly transient and dose-dependent. Although animal models suggested an increased prevalence of medullary thyroid cancer in rats receiving the drug, this is almost certainly not relevant to humans due to a lack of GLP-1 receptor expression in thyroid c-cells compared with an abundance in rodents [51].
We expect phase-3 study results to reproduce these good results, leading to an approval for its use in nondiabetic populations in the next few years.
Exenatide has a similar mechanism of action but is routinely used twice daily due to its shorter half-life and higher peak values [52]. Apparently, it has a similar adverse effect profile, but a smaller weight reduction, although few head-to-head data are available.
Metformin
Metformin, the most-prescribed antidiabetic drug worldwide has a favorable weight profile in this subpopulation. However, the small weight reduction observed with metformin does not make it suitable purely for weight loss, although its use is associated with diabetes reduction risk in the prediabetic population, as seen in the DPP study [53]. Metformin has also been studied in obese children and adolescents, and can be a good option for reducing risk factors [54].
Fluoxetin and Sertralin
Both selective serotonin reuptake inhibitors are antidepressants commonly used for depressive obese individuals. They are not, however, efficient weight-loss medications since the initial weight loss commonly observed is not sustained on a long-term basis, even with continued use [55]. They can be useful for eating disorders such as bulimia nervosa, binge-eating disorder and night eating syndrome as they can reduce compulsive episodes, but the management of these conditions is not within the scope of this chapter.
Bupropion
Bupropion is a norepinephrine and dopamine reuptake inhibitor used mainly for the treatment of depression and smoking cessation [55, 56]. It is proposed that the increased levels of dopamine and norepinephrine in the arcuate nucleus may trigger POMC neuron signaling and lead to appetite suppression [57]. Bupropion alone was studied for the treatment of obesity with a placebo subtracted weight loss of 3–5 kg with an early plateau. This plateau can be explained by a compensatory feedback, with an increased production of beta-endorphin [56].
It has recently been studied in combination with naltrexone (see below) [57].
Combination of Drugs
Before the recent approval of the phentermine/topiramate fixed dose combination, any combination of drugs for the treatment of obesity was considered off-label. It appears, however as an interesting option for its treatment, as we know that it is a multifactorial disease, with linked environmental and genetic factors and redundant pathways that make it difficult to obtain sustained weight loss.
Our group has recently reviewed the possible advantages and disadvantages of combination therapy for the treatment of obesity [58]. Due to synergic or addictive effects it may have increased therapeutic efficacy; it may block compensatory mechanisms that leads to a weight loss plateau; and the potential use of lower doses could minimize adverse effects. Possible disadvantages include costs, drug interactions, and dosage inflexibility (when the combination is in the same pill).
Phentermine and Topiramate Fixed Dose Combination
The rationale for the combination therapy of phentermine and topiramate stems from the good efficacy of both drugs combined with a significant, but very different, profile of adverse effects. As previously cited phentermine can have sympathetic and stimulant effects, as topiramate is more sedative and also reduces blood pressure. In conjunction, both drugs can have additive effects on weight, thus reducing dosages, side effects, non-desired reactions, and discontinuation rates [59].
After good preclinical and clinical studies, a phase 3 trial (CONQUER) evaluated 2,487 individuals over 56 weeks with BMI from 27 to 45 kg/m2 and at least two of the following comorbidities: hypertension, dyslipidemia, diabetes or prediabetes, and abdominal obesity [60]. They were blindly randomized into three groups: placebo, P7.5/T46, and P15/T92 [10]. The median weight loss in absolute and percentage change was 1.4 kg (1.2 %), 8.1 kg (7.8 %), and 10.2 kg (9.8 %), respectively. Patients that completed 1 year of treatment had a mean weight loss of 9.9 kg in the lower dose and an impressive 12.9 kg in the higher dose, making this combination the most potent weight loss drug ever studied in a phase III trial. The percentages of individuals that achieved 5 and 10 % weight loss with placebo, P7.5/T46, and P15/T92 were 21 %/7 %, 62 %/37 %, and 70/48 %, respectively.
It also showed benefits in risk factors, such as glycemia, lipids, and blood pressure and a low dropout rate compared to other weight-loss trials.
An extension Study (SEQUEL Study) evaluated a subgroup of the original participants and observed sustained results with continued use [61].
The most common side effects most significantly related to the drug were dry mouth, paresthesia (the leading cause of discontinuation), constipation, dysgeusia, insomnia, and dizziness, with a dose-dependent pattern. Anxiety and irritability were reported by approximately 4 % of the intervention group versus 2 % in the placebo group. Disturbance in attention was also reported in 4 % of the higher dose group against 2 % in the lower dose and less than 1 % in the placebo group. An increased heart rate of 1.7 beats per minute was seen in the higher dose group, consistent with phentermine’s sympathetic activation. Nephrolithiasis was significantly more frequent in the higher dose group with a 1 % incidence (11 individuals), but there was no difference to blurred vision (a concern with topiramate) between the two intervention groups and placebo [60, 61].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree