Mechanisms of T-Lymphocyte Signaling and Activation
Takashi Saito
INTRODUCTION
The immune system protects us against attack by pathogens through two separate but interacting systems: innate immunity and acquired (or adaptive) immunity. Whereas innate immunity exhibits rapid and transient responses by recognition of conserved molecular patterns of pathogens, adaptive immunity distinguishes any small difference/heterogeneity of a huge number of pathogens and antigens and mediates a rather slow but long-lasting response. For this purpose, as is discussed elsewhere (see Chapter 6) in this volume, our body prepares a vast variety of receptors on T cells and B cells (immunoglobulins [Igs]) to be able to recognize virtually all potential antigens. Because each lymphocyte possesses single antigen specificity, antigen-specific responses begin with the recognition by and activation of a single cell and consequently the cells proliferate, a process called clonal expansion and selection many years ago by Macfarlane Burnet.1 To understand the mechanism of antigen-specific activation of lymphocytes, it is necessary to study how a single cell of relevant specificity recognizes the antigen and induces its activation to mediate various functions and protection against pathogens. Recognition of antigen only on the cell surface by the T-cell receptor (TCR) of a T cell is unique and totally different from that of Ig on a B cell. Such differences in T-cell antigen recognition force the establishment of extensive cell-cell interactions/communications in the body to achieve protective immunity and to maintain immune homeostasis. In this chapter, we describe the unique feature of antigen recognition of T cells through their unique antigen receptor complex and the mechanisms that trigger T-cell activation.
T-CELL RECEPTOR COMPLEX
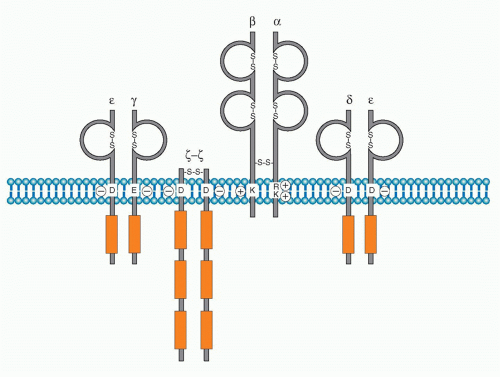
Antigen specific recognition is mediated by the TCR, which is composed of a heterodimer of either αβ or γδ polypeptides. This chapter will focus on signaling mediated through the αβ TCR. As described in Chapter 11, both TCRα and β chains contain variable regions, which together are responsible for binding to the complex of antigen peptide-major histocompatibility complex (MHC). After a decades-long search for the biochemical nature of TCR, the TCR genes were finally cloned and found to be quite similar to those encoding Ig genes.2,3,4,5,6 TCRα and β chains both possess very short cytoplasmic domains without any particular protein-binding motif, and thus cannot transduce antigen-recognition signals into the cell. It is necessary, therefore, for the TCR to assemble with signaltransducing components, the cluster of differentiation (CD)3 complex, to transduce antigen-recognition signals. The initial finding that CD3 chains are responsible for transducing activation signals into T cells came from the observation that treatment of T cells with a crosslinking anti-CD3ε antibody (OKT3) induces strong T-cell polyclonal activation7 similar to that induced upon stimulation with mitogens such as concanavalin A. It was also shown that there is a physical but noncovalent association between TCR dimers and the CD3 complex. It is now known that the association of the TCR dimer and the CD3 complex is required first for cell surface expression and then to transduce antigen-recognition signals into T cells.8,9 The CD3 complex is composed of four different chains. Three of them, γ, δ, and e, are closely related in their protein structure, gene structure, biosynthesis, and chromosome localization. While the γ, δ, and ε are genes are located within a neighboring region of the chromosome (chromosome 11 in human and 9 in mouse), ζ is located on chromosome 1 in both species (Fig. 12.1). The CD3 complex is composed of three distinct dimers, γε, δε, and ζζ, which associate with the TCRαβ dimer. Whereas γε and δε are noncovalently assembled, ζ-ζ is a covalently linked dimmer with a disulfide bond. The ζ chain has an isoform termed η, which is derived from alternative splicing of the ζ transcript and is found only in rodents but not in humans. The cell surface expression of the TCRαβ dimer requires its assembly with the CD3 complex due to a stringent quality control checkpoints in the endoplasmic reticulum (ER) that prevent the release of incompletely assembled TCR-CD3 complexes. Studies of the biosynthesis of these components of the this complex showed that CD3γ, δ, and ε as well as TCRα and β chains are synthesized in great excess and readily degraded in the ER in the absence of assembly with the other chains. The assembly with the partner chain protects the complex from ER degradation. CD3ζ is synthesized at limiting levels, and even the TCRαβ-CD3γδε complex cannot be transferred to the cell surface without assembly with CD3ζζ and is degraded in lysosome.10 Therefore, the assembly with ζ-ζ defines the fully functional TCR-CD3 complex, and the entire TCRαβ-CD3γδεζζ complex is now allowed to transport to the plasma membrane where it is expressed as the complete and functional TCR-CD3 complex.
THE IMMUNORECEPTOR TYROSINE-BASED ACTIVATION MOTIF AND INITIAL SIGNALING
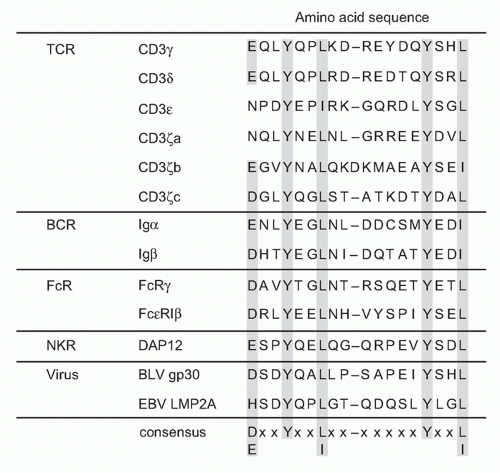
The cytoplasmic tails of all CD3 chains contain a common signaling motif, immunoreceptor tyrosine-based activation motif (ITAM), which has the consensus sequence YxxL/I x6-8 YxxL; composed of two repeats of YxxL/I motif (Y; tyrosine, L/I; leucine/isoleucine) with a spacer of six to eight
amino acids. CD3δ, γ, and ε have a single and CD3ζ has three tandem ITAMs within the intracellular region. The ITAM was first identified as a signature sequence present in several cell surface receptors on immune cells.11 ITAM-containing molecules have since been found to be widely distributed among various important immune receptors, not only TCR (CD3s) but also in B-cell antigen receptors (Igα and Igβ), various types of Fc receptors (FcRγ), adaptor proteins associated with MHC-recognizing receptors, or paired receptors on natural killer (NK) cells (DAP12 and DAP10) (Fig. 12.2) and further in various pattern-recognizing receptors of innate cells such as macrophages and dendritic cells (DCs).12 The ITAM turns out to be specific motif capable of transducing receptor-mediated recognition signals into cellular activation.13 The function of the ITAM in transducing T-cell activation signals has been shown first by analyzing the capacity of a chimeric protein composed of the extracellular domain of CD8 and the intracellular domain of CD3ζ to
induce T-cell activation.14 Antibody crosslinking of the CD8 domain showed that the cytoplasmic tail of CD3ζ can induce almost all the events observed during normal T-cell activation, including tyrosine phosphorylation of various proteins, induction of intracellular calcium mobilization and inositol phosphate metabolism, induction of activation markers, cytokine production, and cell proliferation. Mutational analyses of the ITAM sequences within the CD3ζ cytoplasmic region revealed that both tyrosine and leucine (isoleucine) residues are critical for transducing a T-cell activation signal.15,16
amino acids. CD3δ, γ, and ε have a single and CD3ζ has three tandem ITAMs within the intracellular region. The ITAM was first identified as a signature sequence present in several cell surface receptors on immune cells.11 ITAM-containing molecules have since been found to be widely distributed among various important immune receptors, not only TCR (CD3s) but also in B-cell antigen receptors (Igα and Igβ), various types of Fc receptors (FcRγ), adaptor proteins associated with MHC-recognizing receptors, or paired receptors on natural killer (NK) cells (DAP12 and DAP10) (Fig. 12.2) and further in various pattern-recognizing receptors of innate cells such as macrophages and dendritic cells (DCs).12 The ITAM turns out to be specific motif capable of transducing receptor-mediated recognition signals into cellular activation.13 The function of the ITAM in transducing T-cell activation signals has been shown first by analyzing the capacity of a chimeric protein composed of the extracellular domain of CD8 and the intracellular domain of CD3ζ to
induce T-cell activation.14 Antibody crosslinking of the CD8 domain showed that the cytoplasmic tail of CD3ζ can induce almost all the events observed during normal T-cell activation, including tyrosine phosphorylation of various proteins, induction of intracellular calcium mobilization and inositol phosphate metabolism, induction of activation markers, cytokine production, and cell proliferation. Mutational analyses of the ITAM sequences within the CD3ζ cytoplasmic region revealed that both tyrosine and leucine (isoleucine) residues are critical for transducing a T-cell activation signal.15,16
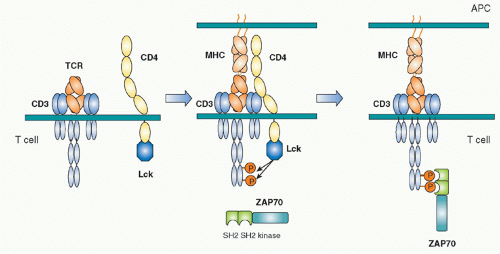
Biochemical analyses to define the downstream signaling molecules recruited upon TCR stimulation resulted in the discovery of several important proteins that are involved in tyrosine phosphorylation and activation of several adaptor molecules including a Src family tyrosine kinase, Lck, and a Syk family kinase, Zeta-associated protein-70 (ZAP-70).17 The ITAM is phosphorylated by Src kinase Lck upon TCR engagement by antigen. Once tyrosines are phosphorylated, they become susceptible to binding in general by proteins possessing Src-homology 2 (SH2) domains. The phosphorylated ITAMs recruit the second tyrosine kinase ZAP-70, one of Syk family kinases. ZAP-70 is contains two tandem SH2 domains in its N terminus and a tyrosine kinase region in the C-terminus.18,19 The tandem SH2 domains with exactly the right spacing can bind the two phosphorylated tyrosines within an ITAM20 (Fig. 12.3). Because TCR and CD3 chains do not have any intrinsic effector function, unlike tyrosine kinase-containing receptors such as the epidermal growth factor receptor or the insulin receptor, the binding of ZAP-70 to the phosphorylated CD3ζ ITAM changes the TCR-CD3 complex, making it competent for signal transduction. ZAP-70 assembled with the CD3 phospho-ITAMs must then be enzymatically activated by phosphorylation of otherwise inhibitory tyrosines in its juxtamembrane region by Lck as well as by itself. The binding and activation of ZAP-70 may be associated with the structural changes of CD3 chains induced by phosphorylation, a topic that will be described under the section Triggering Mechanism. Activated ZAP-70 induces phosphorylation of tyrosine residues of various downstream target molecules.21 These include adaptor molecules LAT and SLP-76. These phosphorylation events lead to further activation of several adaptor and effector molecules and subcellular assembly as multimolecular signaling complex.
REGULATION OF PROXIMAL SIGNALING
Regulation of Tyrosine Kinases
Src family tyrosine kinases (Lck, Fyn in T cells) are composed of an N-terminus unique region, SH2, SH3, and a kinase domain at the C-terminus.22 The N-terminus unique region contains palmitoylation sites by which Lck is localized in lipid rafts on the inner leaflet of the plasma membrane. Because Lck specifically associates with the cytoplasmic regions of CD4 and CD8 coreceptors, Lck accumulates at the site of TCR engagement because CD4 or CD8 are also present within the engaged site through binding to MHC.23,24 As a result, Lck is recruited to the vicinity of the CD3 chains where it is able to phosphorylate them.
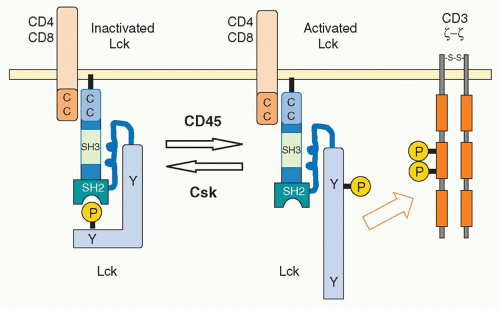
The activity of Lck is regulated allosterically by phosphorylation of a tyrosine in the carboxyl terminus by the
C-terminal kinase (Csk).25 This phosphotyrosine interacts with the SH2 domain of Lck, and this induces a conformational change to maintain Lck in a catalytically inactive status. Csk normally and continuously acts to reduce Lck activity and thereby attenuate TCR signaling. Indeed, in the absence of Csk, peripheral T cells become autonomously activated. Dephosphorylation of the Cterminus tyrosine releases Lck from its inactive status. In addition, in order to fully activate Lck, the tyrosine phosphorylation in the catalytic domain is also required. Because the tyrosine phosphatase CD45 induces the dephosphorylation of both of these regulatory tyrosines, CD45 functions as both a positive and negative regulator of T-cell activation26 through the C-terminal and kinase-domain tyrosines, respectively. The balance between Csk and CD45 regulates the status of T-cell activation27 (Fig. 12.4). A considerable fraction of Lck in naïve T cells is catalytically active because of this dynamic balance. Such active Lck may be responsible for constitutive activation signals including phosphorylation of CD3ζ and help in initiating T-cell activation.28 A related src-family kinase Fyn is also expressed in T cells and is weakly associated with the TCR complex. Fyn appears to have some signaling role because T cells deficient in both Lck and Fyn have a more complete block in T-cell development than Lck-deficient mice.
C-terminal kinase (Csk).25 This phosphotyrosine interacts with the SH2 domain of Lck, and this induces a conformational change to maintain Lck in a catalytically inactive status. Csk normally and continuously acts to reduce Lck activity and thereby attenuate TCR signaling. Indeed, in the absence of Csk, peripheral T cells become autonomously activated. Dephosphorylation of the Cterminus tyrosine releases Lck from its inactive status. In addition, in order to fully activate Lck, the tyrosine phosphorylation in the catalytic domain is also required. Because the tyrosine phosphatase CD45 induces the dephosphorylation of both of these regulatory tyrosines, CD45 functions as both a positive and negative regulator of T-cell activation26 through the C-terminal and kinase-domain tyrosines, respectively. The balance between Csk and CD45 regulates the status of T-cell activation27 (Fig. 12.4). A considerable fraction of Lck in naïve T cells is catalytically active because of this dynamic balance. Such active Lck may be responsible for constitutive activation signals including phosphorylation of CD3ζ and help in initiating T-cell activation.28 A related src-family kinase Fyn is also expressed in T cells and is weakly associated with the TCR complex. Fyn appears to have some signaling role because T cells deficient in both Lck and Fyn have a more complete block in T-cell development than Lck-deficient mice.
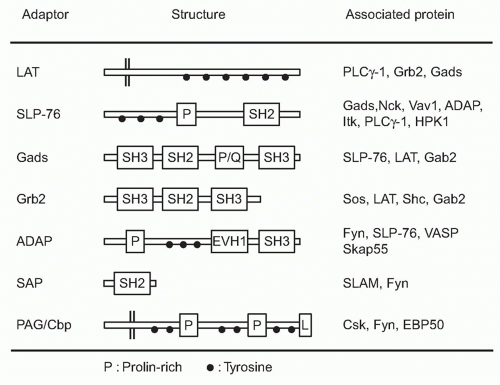
Adaptor-Mediated Signaling
Among the targets of ZAP-70-mediated phosphorylation, a transmembrane adaptor protein, linker for the activation of T cells (LAT),29 and an intracellular adaptor protein, SH2 domain-containing leukocyte phophoprotein of 76 kDa (SLP-76),30 are the most important substrates (Fig. 12.5). The discovery of these two adaptor proteins revealed the connection between the ZAP-70 tyrosine kinase and PLCγ activation. These adaptor proteins create an important signal assembly to induce downstream activation signals. The critical roles of these two adaptors are evidenced by the observation that T cells deficient in either LAT or SLP-76 exhibit complete defects in TCR activation signaling.
LAT is a transmembrane protein with a short extracellular region and resides in lipid rafts, membrane microdomains enriched in cholesterol and sphingolipids, via two palmitoylation sites within the juxtamembrane region. Mutation of these palmitoylation sites results in the failure of LAT to be expressed on the plasma membrane or to transduce T-cell activation, indicating that the association within lipid rafts regulates trafficking to the membrane. Upon TCR stimulation, LAT becomes tyrosine-phosphorylated on multiple tyrosine residues (five tyrosines among nine conserved residues), and these serve as the docking sites for several SH2-containing molecules. Important effector molecules containing SH2 domains that bind to phosphorylated LAT include PLCγ, growth factor receptor binding protein 2 family members, Grb2 and Gads, and a Tec family kinase, inducible T-cell kinase Itk.31 Because Grb2 constitutively binds to son of sevenless (Sos), which is a guanine exchange factor (GEF) that mediates guanine triphosphate (GTP) binding of Ras, this LAT-Grb2-Sos complex induces Ras activation; however, RasGRP plays a more critical role for Ras activation than the Grb2-Sos pathway in T cells. Gads binds to phosphor-LAT upon T-cell activation, and Gads associates with SLP-76. Although all tyrosine mutations of LAT lost the function for T-cell activation, the LAT136 mutant mouse exhibits a lymphoproliferative disorder and induction of strong Th2-type cytokine production, suggesting the possibility of a LAT-independent pathway to activate T cells.32,33
SLP-76 is a cytosolic adaptor with three domains: an amino terminal region containing three major tyrosines that are phosphorylated upon TCR ligation and become binding sites for various SH2-containing proteins, a central proline-rich domain that binds to SH3-containing proteins, and a carboxy-terminal SH2 domain by which SLP-76 can bind to phosphorylated tyrosines of other proteins.34 SLP-76 constitutively associates with Gads through its SH3 region, and the SLP-76/Gads complex is recruited to the phosphorylated LAT upon TCR engagement (Fig. 12.6). Both LAT and SLP-76/Gads bind to PLCγ1 independently, and formation of this tetramolecular assembly stabilizes the complex. Because LAT also binds to the Itk Tec family kinase,
which is responsible for phosphorylating PLCγ1, the stable complex of Itk-LAT-SLP-76-PLCγ1 induces PLCγ1 activation. Besides PLCγ1 activation, SLP-76 induces other functions through its association with several other important molecules. At its N-terminus, SLP-76 binds to Vav, a GEF, and Nck, an adaptor protein, both of which are critical for TCR-mediated cytoskeletal changes, and at its C-terminus it binds adhesion- and degranulation-promoting adapter protein (ADAP),35,36 an adapter protein coordinating TCR signals with integrin activation (see Fig. 12.12).
which is responsible for phosphorylating PLCγ1, the stable complex of Itk-LAT-SLP-76-PLCγ1 induces PLCγ1 activation. Besides PLCγ1 activation, SLP-76 induces other functions through its association with several other important molecules. At its N-terminus, SLP-76 binds to Vav, a GEF, and Nck, an adaptor protein, both of which are critical for TCR-mediated cytoskeletal changes, and at its C-terminus it binds adhesion- and degranulation-promoting adapter protein (ADAP),35,36 an adapter protein coordinating TCR signals with integrin activation (see Fig. 12.12).
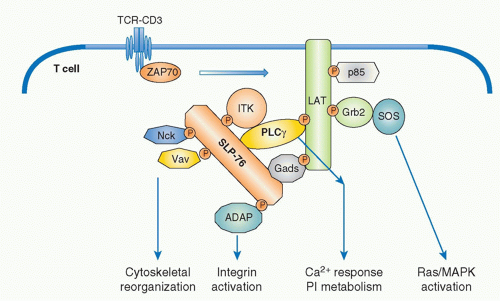 FIG. 12.6. Formation of Multimolecular Signaling Complex Critical for Initial T-Cell Receptor (TCR) Activation Signaling Pathways. Sequential activation of two tyrosine kinases Lck and ZAP-70 upon antigen recognition by TCR induces phosphorylation of two critical adapter proteins LAT and SLP76. Gads constitutively associates with SLP76 and is phosphorylated by TCR activation and recruited to LAT as the Gads-SLP76 complex. Thereafter, several adapter proteins form a physical complex centralized by LAT and SLP-76. Further recruitment of various adapters and effector molecules to the complex leads to the activation of differential signaling pathways. Phospholipase γ1 (PLC-γ1) and Gads link between LAT and SLP76 and a Tec-kinase family ITK, which binds to SLP76, is responsible to phosphorylate/activate PLC γ1. Association of these molecules generates a stable complex of LAT-Gads-SLP76-PLCγ1-ITK critical for further calcium and phosphatidylinositol (PI) responses (as in Fig. 12.7). SLP76 also binds Nck, Vav1, and ADAP, which links to cytoskeletal reorganization (as in Fig. 12.11). LAT binds Grb2/Sos, which induces Ras/MAPK activation (as in Fig. 12.8). |
DOWNSTREAM SIGNALING PATHWAYS
The next step of the T-cell activation pathway after ZAP-70-induced phosphorylation of the adaptor LAT and SLP-76 upon TCR ligation is the activation of the key signaling enzyme PLCγ1. PLCγ1 is recruited to the plasma membrane through the binding of its PH domain to PIP3, which is generated within the membrane by the phosphorylation of PIP2 by PI3-kinase. PLCγ1 is activated upon phosphorylation by Itk,37,38 which is composed of PH, SH2, SH3, and kinase domains, and is also recruited to the plasma membrane through its PH domain by interacting with PIP3. Phosphorylated and activated PLCγ1 then cleaves the membrane bound PIP2 to generate two critical products: the membranebound lipid diacylglycerol (DAG) and the diffusible inositol 1,4,5-triphosphate (IP3), both of which function as second messengers for further inducing downstream signaling.
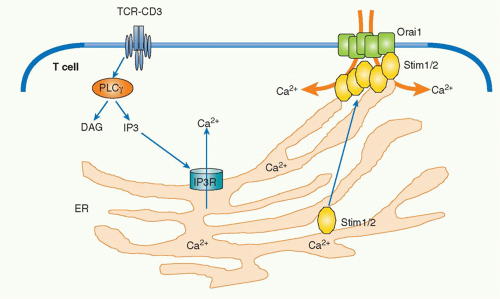
Calcium-Nuclear Factor of Activated T Cells
Upon TCR stimulation, the levels of intracellular free calcium are regulated in two phases: there is an initial and transient induction of calcium release from storage in the ER, and this is followed by the induction of a strong influx of high levels of calcium from outside of the cells by opening a calcium channel called the calcium release-activated calcium channel (CRAC) in the plasma membrane. IP3 generated by PLCγ1 catalysis diffuses into the cytoplasm and binds to IP3 receptors on the ER membrane. IP3 receptors are calcium channels and, after IP3 binding, they open and allow the release of the calcium stored within ER into the cytosol. The released low level of calcium induces cluster formation by the calcium-binding transmembrane protein, stromal interaction molecule-1 (STIM1), within the ER.39 STIM1 is an ERresidential protein that contains an N-terminal sterile motif and paired EF hands that function as a calcium-binding motif and a coiled-coil motif at the C terminus. A critical CRAC called Orai1 has been identified from the analysis of a patient with severe combined immunodeficiency who had a major defect in lymphocyte activation.40 Clustered STIM1 on the ER membrane is colocalized and assembled with Orai1 on the nearby plasma membrane to open the channel and introduce extracellular calcium into the cytosol (Fig. 12.7). Recent analysis has shown that oligomerization of STIM1 is sufficient to induce CRAC activation independent of ER calcium store depletion, but the mechanism by which STIM1 induces Orai1 oligomerization remains unknown.
Surprisingly, STIM1, STIM2 (another family member), and Orai1 are dispensable for T-cell development as deficiency of these molecules does not affect this process. Because calcium flux is also induced and is important in thymocytes, it is assumed that there must be other functionally redundant and critical calcium channels in thymocytes.41
Surprisingly, STIM1, STIM2 (another family member), and Orai1 are dispensable for T-cell development as deficiency of these molecules does not affect this process. Because calcium flux is also induced and is important in thymocytes, it is assumed that there must be other functionally redundant and critical calcium channels in thymocytes.41
Upon T-cell engagement, intracellular calcium binds to a protein, calmodulin, and induces a conformational change that allows the protein to bind to various target proteins. An increase of cytosolic calcium induces activation of signaling molecules including calcium calmodulin-dependent kinase and a phosphatase calcineurin. Activated calcineurin acts on a critical transcription factor, nuclear factor of activated T cells (NFAT), to dephosphorylate the protein, which induces its translocation into the nucleus.42 There are five NFAT family members (NFATc1, c2, c3, c4, and NFAT5), and they are expressed in many different tissues.43 NFAT is present in the cytoplasm in the resting state in T cells. Cytosolic serine/threonine kinases such as glycogen synthase kinase 3 and casein kinase 2 phosphorylate the nuclear localization signal on NFAT to prevent its translocation into nucleus. Upon antigen stimulation, activated calcineurin dephosphorylates this critical phosphorylation site, which allows NFAT to enter into the nucleus, where it triggers various gene expression programs including various cytokine genes by cooperating with other transcription factors. The most well-characterized gene among NFAT targets is the interleukin (IL)-2 gene. NFAT binds to the IL-2 promoter by forming a cooperative complex with activator protein (AP)-1, which is a heterodimer composed of members of the Jun and Fos family, to induce IL-2 transcription. NFAT and AP-1 represent two signaling pathways of calcium and Ras/MAPK, respectively. It has been shown that T-cell activation in the absence of the induction of AP-1 results in anergic or unresponsive T cells.44
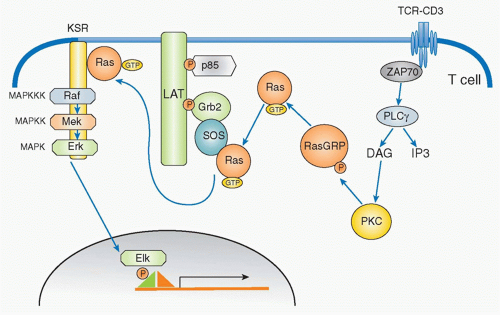
Ras-Mitogen-Activated Protein Kinase
In parallel with IP3, DAG is generated by PLCγ and remains localized within the plasma membrane where it recruits various signaling molecules that contain a specific DAG-binding motif (called the C1 domain), including members of the protein kinase C (PKC) family45 and RasGRP,46 a guaninenucleotide exchange factor for Ras. DAG binding activates PKC and promotes RasGRP to activate Ras by exchange from the guanosine diphosphate (GDP) to GTP-binding form.47 Ras is active in the GTP-bound form, and this activation step is mediated by GEFs and suppressed by GTPaseactivating proteins (GAPs). RasGRP and Sos are two major GEFs in the TCR activation pathway (Fig. 12.8). In T cells, RasGRP functions dominantly for early activation of Ras. RasGRP is inducibly associated with the plasma membrane through binding of its C1 domain to DAG and is phosphorylated by PKCθ. On the other hand, Sos constitutively associates with an adaptor protein Grb2 and the Grb2 SH2 domain binds to phosphorylated LAT upon T-cell activation; Sos-Grb2 binds to LAT-SLP76, which results in its recruitment to
the vicinity of the TCR. The relationship between RasGRP and Sos appears to be cooperative rather than competitive as RasGRP-mediated Ras activation enhances Sos activity as a positive feedback to induce strong Ras activation.48
the vicinity of the TCR. The relationship between RasGRP and Sos appears to be cooperative rather than competitive as RasGRP-mediated Ras activation enhances Sos activity as a positive feedback to induce strong Ras activation.48
Ras activation triggers a cascade of kinase activation, which finishes by activating a serine/threonine kinase known as a mitogen-activated protein kinase (MAPK). The MAPK cascade is composed of three kinases: MAPK kinase kinase (MAP3K), MAPK kinase (MAP2K), and the MAPK itself. In the case of the TCR signaling pathway, the first MAP3K is Raf, which is a serine/threonine kinase that phosphorylates the next MAP2K, which in T cells is MEK1. MEK1 is called a dual-specificity kinase because it can phosphorylate both a tyrosine and a threonine of the last member of the cascade, the MAPK extracellular signal-regulated kinase 1 (Erk1) and Erk2 in T cells and B cells, respectively. Erk1/2 induces the activation of Elk1, which in turn activates the AP-1 transcription factor complex composed of Fos and Jun.
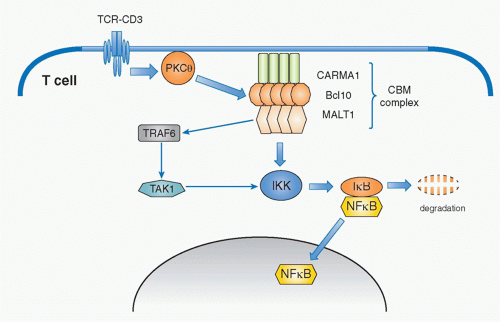
PKC-CARMA1/Bcl-10/Malt1-Nuclear Factor of KappaB
Historically, it has long been known that phorbol ester (phorbol 12-myristate 13-acetate) plus a calcium ionophore, a molecule that allows calcium ions to cross cell membranes, induce strong activation signals similar to TCR-induced signals.49 Phorbol ester is known to activate PKC; thus, PKC is a critical component in T-cell activation. T cells predominantly express the PKCθ isoform of protein kinase C. PKCθ has a DAG-binding domain, is recruited to the plasma membrane through binding to DAG, and is activated upon generation of DAG by PLCγ1. One of the main pathways activated by PKCθ is NF-κB. PKCθ plays a critical role in initiating several cascades of the classical pathway of NF-κB activation downstream of the TCR. NF-κB is a family of transcription factors composed of homo- and heterodimers of five members of the Rel family including NF-κB1 (p50), NF-κB2 (p52), RelA (p65), RelB, and c-Rel (Rel).50 In resting T cells, NF-κB is found in the cytosol associated with one of a family of inhibitors of NF-κB (IκB) that prevents NF-κB from translocating into the nucleus. Upon T-cell activation, IκB is phosphorylated by the IκB kinase (IKK) complex, ubiquitinylated and degraded, which allows NF-κB to translocate into nucleus, where it activates various genes involved in survival, homeostasis, and activation of T cells and inflammatory responses.
Whereas this signaling pathway to activate NF-κB is common among many cell types and has been known for quite some time, the specific pathway by which PKCθ induces NF-κB activation has only been elucidated in the past decade. The critical molecule responsible for mediating the activation signal that connects PKCθ and NF-κB is now known as the CBM complex (Fig. 12.9). It consists of three proteins; a scaffold protein, CARMA1 (caspase recruitment domain [CARD] and membrane-associated guanylate kinase -containing scaffold protein), a CARD-containing
adaptor protein, B-cell lymphoma 10 (Bcl10), and mucosaassociated lymphoid tissue lymphoma translocation gene 1 (Malt1).51,52,53 Upon TCR stimulation, this trimolecular complex is induced by PKCθ-mediated phosphorylation of CARMA1, which is required for CARMA1 oligomerization and association with Bcl10. Malt1 binds to Bcl10 and mediates the degradation of the IKKγ subunit (also called NEMO) by inducing polyubiquitination through activation of an E3 ubiquitin-ligase, tumor necrosis factor receptor-associated factor 6 (TRAF6).55,56 TAK1 recruited by TRAF6 phosphorylates IKK, which in turn induces IκB phosphorylation. This event, together with degradation of NEMO, induces consequently IκB degradation and release and translocation of NF-κB to the nucleus, and thereby induces gene activation.
adaptor protein, B-cell lymphoma 10 (Bcl10), and mucosaassociated lymphoid tissue lymphoma translocation gene 1 (Malt1).51,52,53 Upon TCR stimulation, this trimolecular complex is induced by PKCθ-mediated phosphorylation of CARMA1, which is required for CARMA1 oligomerization and association with Bcl10. Malt1 binds to Bcl10 and mediates the degradation of the IKKγ subunit (also called NEMO) by inducing polyubiquitination through activation of an E3 ubiquitin-ligase, tumor necrosis factor receptor-associated factor 6 (TRAF6).55,56 TAK1 recruited by TRAF6 phosphorylates IKK, which in turn induces IκB phosphorylation. This event, together with degradation of NEMO, induces consequently IκB degradation and release and translocation of NF-κB to the nucleus, and thereby induces gene activation.
Overexpression of the CBM complex induces spontaneous NF-κB activation without T-cell (or B-cell) activation. Recently, it has been shown that a high proportion of diffuse large B-cell lymphomas are induced by specific oncogenic mutations of CARMA1, which induce its spontaneous oligomerization of CARMA1, formation of the CBM complex, and subsequent continuous chronic activation of NF-κB, ultimately resulting in tumor induction.57 This illustrates the importance of maintaining appropriate control of the CBM complex-mediated NF-κB activation for appropriate immune responses without inducing excess inflammation and diseases.
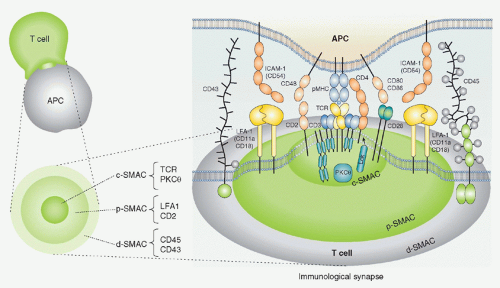 FIG. 12.10. Schematic Structure of the Immunologic Synapse. Antigen recognition and activation of T cells starts by the specific interaction between T cells and antigen-presenting cells (APCs) (left). In the interface of two cells, special structure with segregated arrangement of receptors and signaling molecules is induced where T-cell receptros (TCRs) and major histocompatibility complexes (MHCs) are accumulated in the central region termed as the central supramolecular activation cluster (SMAC), the adhesion molecule leukocyte function-associated antigen (LFA)-1-intercellular adhesion molecule 1 is localized at the surrounding area around the TCR-MHC (as the peripheral SMAC), and further large molecules such as cluster of differentiation (CD)45 are located outside peripheral region SMAC as distal SMAC (right). This “bulls eye-like” structure is dynamically induced after T cell-APC contact; LFA-1 moves outside and the TCR-CD3 moves into the center within 10 minutes. Activation signals are induced not at the synapse but at TCR microclusters, which are generated at the interface between T cell and APCs prior to central SMAC formation, which is composed of TCR, kinases, and adaptors.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|