, Hans Guski2 and Glen Kristiansen3
(1)
Carl-Thiem-Klinikum, Institut für Pathologie, Cottbus, Germany
(2)
Vivantes Klinikum Neukölln, Institut für Pathologie, Berlin, Germany
(3)
Universität Bonn, UKB, Institut für Pathologie, Bonn, Germany
11.1 Diagnostic Antibody Panel for Tumors of the Vulva and Vagina
Cytokeratin profile, p63, CEA, p16, HPV, steroid hormone receptors, desmin, myogenin, and melanoma markers.
11.2 Diagnostic Antibody Panel for Tumors of the Uterine Cervix
Cytokeratin profile, p63, CEA, PAX-8, PAX-2, p16, p53, HPV, and steroid hormone receptors.
11.3 Diagnostic Antibody Panel for Epithelial Tumors of the Uterine Corpus, Fallopian Tube, and Uterine Ligament
Cytokeratin profile, CEA, PAX-8, p16, p53, HNF-1β, and steroid hormone receptors.
11.4 Diagnostic Antibody Panel for Uterine Mesenchymal Tumors
Smooth muscle markers, CD10, and steroid hormone receptors.
p16 | ||
|---|---|---|
Expression pattern: nuclear/cytoplasmic | ||
Main diagnostic use | Expression in other tumors | Expression in normal cells |
HPV-associated oropharynx and uterine cervix squamous cell carcinoma, atypical lipomatous tumors and liposarcoma | Endometrial serous carcinoma, clear cell carcinoma, melanocytic nevi and melanoma, adenoid cystic carcinoma, malignant mesenchymal tumors | |
Positive control: cervical squamous cell carcinoma | ||
Diagnostic Approach
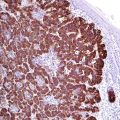
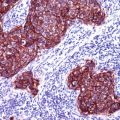
P16 (also known as INK4a or cyclin-dependent kinase inhibitor 2A) is a tumor suppressor protein encoded by the p16INK4a gene. p16 inhibits the cyclin-dependent kinases [1, 2] involved in in cell cycle regulation and progression (G1 to S). p16 plays role in the pathogenesis of different malignancies. The expression of p16 is regulated by the retinoblastoma (Rb) gene, which in turn is affected by the E7 oncogene of the HPV gene. p16 is overexpressed in HPV-associated intraepithelial dysplasia and squamous cell carcinomas of different origins including vulvar, vaginal, and cervical squamous cell carcinoma in addition to oropharynx carcinoma. In routine immunohistochemistry, p16 reveals cytoplasmic and nuclear staining pattern and the intensity of the stain correlates with grade of HPV infection and grade of associated dysplasia. p16 is also highly expressed in uterine serous carcinoma and a helpful marker that labels the cells of serous tubal intraepithelial carcinoma (STIC) [3].
PAX-8:
PAX-8 is a transcriptional factor involved in the fetal development of the brain, eye, thyroid tissue, kidney, and upper urinary system as well as the Müllerian organs. PAX-8 is listed in detail in a next chapter.
Hepatocyte Nuclear Factor-1β (HNF-1β):
HNF-1β is a member of the hepatocyte nuclear factor family regulating the growth and differentiation of hepatocytes and cells of the biliary system. The expression of different hepatocyte nuclear factors is not restricted to the liver but variously found in other organs including the pancreas, kidney, prostate, and female genital system [6]. HNF-1β is used in diagnostic immunohistochemistry to differentiate between different types of ovarian and endometrial carcinomas. The strong nuclear HNF-1β expression is characteristic for both endometrial and ovarian clear cell carcinomas but usually negative in reactive lesions with clear cell appearance such as clear cell metaplasia and Arias-Stella phenomenon [7]. However, we must consider that focal weak to moderate HNF-1β expression can be also found in other endometrial and ovarian carcinoma types such as endometrioid and serous carcinomas [8]. Additionally, different HNF-1β expression intensity is also found in other carcinomas of different origin including colorectal, pancreatobiliary, prostatic, and renal cell carcinomas.
Phosphatase and Tensin Homolog (PTEN):
PTEN is a widely expressed enzyme in mammalian cells that catalyzes the dephosporylation of the 3` phosphate of the inositol ring, an essential reaction that causes the inhibition of the protein kinase (AKT) signaling pathway involved in the regulation of apoptosis. Mutations that inactivate the PTEN gene cause the inhibition of the apoptotic cascade increasing cell proliferation. Inactivating mutations within the PTEN are commonly seen in different human neoplasias such as urogenital, breast, and lung carcinomas in addition to melanoma and glial tumors [9]. The immunohistochemical staining of PTEN (cytoplasmic pattern) is a simple way to detect the loss of this enzyme. The loss of PTEN expression is found in 30–50% of endometrial carcinoma and in about 25% of endometrium with atypical complex hyperplasia, which indicates that the loss of PTEN is not a specific marker of malignant transformation [10, 11]. Normal proliferative endometrium shows usually strong PTEN expression. The loss of PTEN expression is also found in a subset of ovarian endometrioid carcinoma (~20%), high-grade serous carcinoma, and clear cell carcinoma.
A fraction of high Gleason prostatic carcinoma is also associated with PTEN loss (see markers of prostatic carcinoma) [9]. PTEN mutations are found in primary glioblastoma but rare in secondary glioblastoma.
Steroid Receptors:
Both estrogen and progesterone receptors were discussed in details with the markers of breast tumors. Endometrial adenocarcinoma and serous endometrial carcinoma are sex hormone-dependent tumors, and the expression of estrogen and progesterone is characteristic for both carcinoma types [12]. Myometrium is also a target tissue for steroid hormones; accordingly the majority of uterine leiomyomas and leiomyosarcomas are positive for estrogen receptors, progesterone receptors, or both. This characteristic feature can be used to differentiate between uterine and soft tissue leiomyosarcoma [13]. Squamous cell carcinoma and adenocarcinoma of uterine cervix usually lack the expression of both receptors [14].
Immunoprofile of tumors of the uterine cervix, uterine corpus, and fallopian tube | ||||
|---|---|---|---|---|
Tumor type | + in >90% (+) | + in 50–90% (±) | + in 10–50% (∓) | + in <10% (−) |
A. Tumors of the vulva and vagina | ||||
Paget’s disease of the vulva | CK7, EMA (MUC1), CEA, androgen receptors | ER | GCFP-15 | CK5/6/14, CK20 |
Squamous cell carcinoma | CK5, CK6, CK18, CK19, P16 | CK7, CK20 | ||
Bartholin gland carcinoma • Adenocarcinoma • Squamous cell carcinoma • Adenoid cystic carcinoma • Transitional cell carcinoma | See immunoprofile of similar carcinomas of other locations | |||
Adenocarcinoma of mammary type | See immunoprofile of breast carcinoma | |||
Adenocarcinoma of Skene gland type | Pan-CK, PSA | PAX-8 | ||
Clear cell carcinoma | CK7, EMA, CEA | CK20 | ||
Sebaceous carcinoma | Adipophilin, EMA, androgen receptors | Perilipin, CK5/14, CK8/18, CK7, CK19, CD15, p16 | CK20, CEA, S100 | |
Angiomyofibroblastoma | Desmin | ER, PgR | CD34 | Actin |
Cellular angiofibroma | CD34, ER, PgR | Actin | ||
Superficial angiomyxoma | CD34 | Actin, desmin, S100 | ||
Deep aggressive angiomyxoma | Desmin, HMGA2 | Actin, ER, PgR | CD34, actin, S100 | Myogenin, MyoD1 |
Epithelioid sarcoma | See miscellaneous soft tissue tumors | |||
Rhabdomyosarcoma | See soft tissue rhabdomyosarcoma | |||
B. Tumors of the uterine cervix | ||||
Squamous cell carcinoma of the cervix and uterus | CK5, CK6, CK13, CK17, CK18, CK19, P16 | CK14 | CK7, CK20, ER, PgR | |
Endocervical adenocarcinoma | CK7, CK8, CK18, CK19, CEA, EMA, p16, PAX-8 | CK20, vimentin | ER, PgR, CK5/6, WT-1, PAX-2a, GFAP | |
Endometrioid adenocarcinoma | CK7, CK8, CK18, CK19, EMA | ER, PgR, vimentin, GFAP | p16, CD56 | CK20, CK5/6, CEA, CDX-2 |
Mesonephric adenocarcinoma | CK5/6, CK7, CK8, CK18, EMA, CD15 | CD10, p16, calretinin, vimentin, bcl-2 | Androgen receptors, PAX-8, TTF-1 | ER, PgR, CK20, CEA |
Adenosquamous carcinoma/glassy cell carcinoma | CK7b, CK5/6/14c | ER, PgR | ||
Adenoid basal carcinoma | CK5/14, p63, p16 | |||
Neuroendocrine tumors • NET(c) G1 • NET(d) G2 • NEC(e) G3 (small cell carcinoma)j, k, l | Pan-CK, CD56, NSE, PGP9.5 Proliferation index (Ki-67) in NET G1: <2% NET G2: 3–20% NEC G3: >20% | Synaptophysin, chromogranin | TTF-1 | CK7, CK20 |
C. Tumors of the uterine corpus | ||||
Endometrial adenocarcinoma | CK7, CK8, CK18, CK19, PAX-8, EMA, CA125 | PgR, ER, vimentin, GFAP | CD56, p53, P16 | CK20, CK5/6, CEA, WT-1, IMP3, CDX-2d |
Serous endometrial carcinoma | CK7, CK8, CK18, CK19, EMA, CA125, p16, p53, PAX-8, β catenin Proliferation index (Ki-67): >75% | IMP3, PgR, ER | ER, PgR, Sox-2, WT-1 | CK5/6, CK20, HNF1-β |
Clear cell carcinoma | CK 7, EMA, CA125, PAX-8, hepatocyte nuclear factor 1-β (HNF1-β), p504s (AMACR) | Vimentin, CD15 | ER, AFP, CEA, p16, p53, Sox-2 | PgR, WT-1, CK20, CD10 |
Undifferentiated carcinoma | EMA, vimentin | Pan-Cytokeratin, CK8/18, p53 | PAX-8, synaptophysin, chromogranin | ER, PgR |
Low-grade endometrial stromal sarcoma
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||