Chapter 35 Managing Pregnant Patients
INTRODUCTION
The AIDS epidemic continues to affect many millions of people globally. UNAIDS estimated that there were 39.5 million people living with HIV in 2006, of which 17.7 million (almost half) were women and 2.3 million children.1 More than 75% of these women live in sub-Saharan Africa (SSA), which is also home to most of the 700 000 new infections in children each year. Central Asia and Eastern Europe continue to see increases in new infections in women, while the epidemic appears to have stabilized more in Western Europe and the United States. In Western Europe, an increasing proportion of heterosexual infections are diagnosed in immigrants, particularly from SSA,2–4 as may be expected with the higher prevalence rates in these countries of origin. This may impact on the care of pregnant women if they do not have full access to HIV and maternity care services.
In the United States, the absolute number of infections has decreased from a peak in 1992.5 While most cases continue to be seen in men, the proportion of infections in women has risen from 15% in the period 1981–95 to 27% in the period 2001–04.6 This correlates with an increase in the heterosexual mode of transmission from 10% to 30%. An additional racial skewing of the epidemic has been seen in the United States with 51% of new cases in 2001–04 occurring in blacks with a case rate in black women 21 times higher than that seen in white women (67 per 100 000 compared to 3.2 per 100 000). Although HIV infection in pregnancy is less common in some areas of the United States, these changes in the epidemiologic pattern mean that it continues to be a major concern in others.
The differences in HIV prevalence between developed and less developed countries are also seen in the rates of mother-to-child transmission (MTCT) of HIV. Although 700 000 infected children are born in the world each year, more than a decade of advances in MTCT prevention strategies has resulted in a reduction of infections in the US and Europe which make pediatric HIV infection there a relatively rare event. Transmission rates in the US have dropped to below 2%, with an estimate of only between 144 and 236 infected children born in the US in 2002.7 Put another way, one HIV-infected child is born every minute in the less developed parts of the world, less than one a day in the most developed countries.
The counseling and care of HIV-infected pregnant women must consider the impact of the pregnancy on HIV disease, maternal health and choice of therapy, and the effect of HIV on pregnancy outcome, including the risk of MTCT of HIV. The mother’s life expectancy, her longer-term parenting plans and the follow-up needs of the infant should be discussed in the counseling. The benefits and risks of specific treatments should be discussed, and recommendations for starting or continuing antiretroviral therapy during pregnancy must take into account the woman’s choice, previous or current antiretroviral therapy, gestational age, maternal CD4+ T-lymphocyte count and HIV RNA level, and the potential toxicities‥ Guidelines for the use of antiretrovirals in pregnancy are updated frequently, as new information becomes available, and are available on the World Wide Web, and health providers caring for HIV-infected pregnant women are advised to consult the most current applicable guidelines for up-to-date information.8–10
IMPACT OF PREGNANCY ON MATERNAL HEALTH
Maternal Health and HIV
Most data from the United States and Europe suggest that pregnancy does not accelerate HIV disease progression.11–16 A similar situation has been described in Thailand.17 Although a systematic review and meta-analysis of seven cohort studies in HIV-infected women from 1983 to 1996 suggested a weak association between death, disease progression and progression to an AIDS-defining illness and pregnancy, this association was stronger in the one study conducted in a resource-poor setting.18 In this meta-analysis, the summary odds ratios for the risk of an adverse maternal outcome related to HIV infection and pregnancy were 1.8 (85% confidence interval (CI), 0.99–3.3) for death, 1.41 (95% CI, 0.85–2.33) for HIV disease progression and 1.63 (95% CI, 1.00–2.67) for progression to an AIDS-defining illness, although this research reflects the situation prior to widespread use of highly active antiretroviral therapy (HAART). Data from the US Women and Infants Transmission Study (WITS) over a 12-year period from 1989 to 2002 showed a 70% reduction in progression to death following AIDS associated with HAART.19 Minkoff and colleagues studied the effect of pregnancy on progression of HIV disease in 953 women in the United States who had no additional pregnancy after the index pregnancy, compared with 329 women who had a second pregnancy subsequent to their index pregnancy. They showed no compared significant differences in the immunologic, clinical, and virologic courses of women with a subsequent pregnancy, compared to those without.20 In a more recent cohort of 786 women with access to HAART, pregnancy was associated with a lower rate of progression to AIDS defining illnesses.21 No benefit to maternal health has been demonstrated by pregnancy termination.
CD41 T-lymphocyte counts drop during pregnancy in both HIV-infected and uninfected women, but will generally rebound in the postpartum period.22–26 The drop in CD4+ counts may be more pronounced in women not receiving HAART than in those on therapy. One study in Ireland showed a decrease in CD4+ counts of 27% in those not on HAART compared to 13% on HAART.27 The pregnancy related decrease in CD4+ count may impact on treatment decisions where CD4+ count is one of the eligibility criteria for HAART, especially where pregnant women who do not qualify for ongoing HAART will otherwise receive only shorter antiretroviral regimens for prevention of MTCT (PMTCT). This may result in “over treatment” of a significant number of women whose CD4+ counts would return to higher levels postpregnancy. It has been suggested that CD4+ percentage is less variable during pregnancy and may be a more reliable or robust measure than absolute CD4+ count in pregnant women.28 At present, most national and international guidelines have not recommended changing to use of CD4+ percentage, and continue to use absolute CD4+ counts for treatment decisions.
HIV infection may be associated with reduced fertility, in reports from Africa and from resource-rich settings.29–36 Birth intervals are longer in HIV-positive women in some studies, and a prolonged time to achieve conception has been associated with higher viral load.37,38 A prospective Ugandan study showed lower rates of pregnancy and more pregnancy loss in HIV-positive compared to HIV-negative women.39 Lowered fertility may also be associated with menstrual abnormalities in severely ill positive women with weight loss.40
Maternal Mortality and HIV
In developed countries the availability of specialist HIV treatment and care in pregnancy makes HIV a rare cause of maternal mortality. Pregnancy may impact more on the progression and outcome of HIV infection in less resourced settings, leading to more rapid progression to AIDS or death.41,42 Although specific data on disease progression in pregnancy is limited, there is evidence from several studies in Africa that HIV has become a significant contributing cause of maternal mortality.43–48 HIV/AIDS related conditions may worsen postpregnancy, manifesting in the first year after delivery and thus may be underestimated in official maternal mortality figures.49 This postpregnancy mortality is demonstrated in several reports. In Zaire, the maternal mortality rates in HIV-infected women were ten times those of HIV-negative women50; with 22% of HIV-infected mothers dying during a 3-year follow-up period. In a prospective study in Malawi, the maternal mortality rate was 370 per 100 000 women and the mortality rate between 6 weeks and 1 year postpartum was 341 per 100 000 live births, with AIDS and anemia the major causes of postpregnancy mortality.51
The risk of more rapid HIV progression during and after pregnancy may be influenced by multiple factors, including nutritional and maternal genetic factors. Progression postpregnancy may be more rapid in women who have anemia during pregnancy52 and delayed by the administration of multivitamin supplements.53 A Kenyan study demonstrated that an increase in mortality postpregnancy was correlated with CCR5 promoter polymorphisms. This study showed a three-fold increase in death within 2 years in women (95% CI, 1.0–9.5) with the 59356 C/T genotype compared with women with the 59356 C/C genotype.54
In high prevalence areas, the impact of HIV on maternal mortality may be mitigated by the effect of reduced fertility in HIV-infected women. In a situation where fewer HIV-positive women become pregnant, HIV-linked mortality may be reduced or maternal mortality figures may even decline. As evidence of this, a study in the Congo has shown a 32 times higher mortality rate in HIV-infected women than uninfected, but a higher relative increase in nonpregnant than pregnant women.55 In these settings, maternal mortality also impacts on child survival, with children more likely to die if their mothers die.56–58 This may negate any improvements in survival of children achieved by PMTCT programs unless appropriate antiretroviral treatment for mothers is also instituted.
MTCT OF HIV
The development and implementation of strategies to prevent MTCT of HIV represents one of the most successful responses to the AIDS epidemic. Within the space of a decade, from 1994, perinatal HIV infection has been dramatically reduced in the United States, Europe and other developed world settings, and less expensive, more feasible regimens have been successfully used in less-resourced settings. Transmission rates in the US and Europe have dropped to less than 2%, with the widespread access to interventions for the PMTCT of HIV.7 Despite this progress, more remains to be done to make interventions available to all of the 2 million HIV-infected pregnant women globally each year. Worldwide, 1800 children are infected each day and UNICEF estimates that a child dies every minute of every day from HIV infection, almost all of which is transmitted from mother to the child.1,59 UNAIDS estimates that less than 8% of pregnant women worldwide are offered PMTCT services, dropping to under 6% in SSA, and that only 9% of HIV-infected pregnant women worldwide received antiretroviral prophylaxis for PMTCT in 2006.1 In the 33 most affected countries, an estimated 26% of children born to HIV-infected mothers were themselves infected in 2005. Although this represents a 10% decrease from the estimated transmission rate in 2001, it illustrates the huge gap between the situation in these countries and the USA or Europe.
Factors Affecting MTCT
MTCT of HIV can occur in utero, during labor and delivery or through breast milk, although the bulk of transmission occurs during the intrapartum period.60,61 These proportions of infection will change according to the availability of prevention interventions. Treating with HAART throughout pregnancy will almost eliminate antepartum and intrapartum transmission, while short-course antiretroviral interventions will have little effect on in utero transmission, but can dramatically reduce intrapartum transmission.62,63 In nonbreastfeeding populations, about one-third (ranging from 20% to 60%) of transmissions appear to occur in utero, with the remaining two-thirds (from 40% to 80%) occurring during labor and delivery.64–69 Evidence suggestive of intrapartum transmission includes delayed detection of HIV by culture, antigen detection, or DNA in the neonate;66–68 later onset of clinical symptoms;65 detection of HIV in cervicovaginal secretions of infected women that is reduced after antiretroviral therapy and has been shown to reduce transmission;69–71 significantly higher transmission rates in the first-born twin compared to the second-born twin;72 the association of higher transmission rates with increasing duration of ruptured membranes during labor;73–75 and reduced transmission among women delivered by cesarean section before labor and membrane rupture.76,77
The risk of MTCT varies by maternal stage of disease, high viral load, low CD4+ count, use of antiretroviral therapy, mode of delivery, duration of rupture of membranes, increased genital secretion of HIV, the practice of breastfeeding and other factors such as prematurity (Table 35-1).
Table 35-1 Risk Factors for Perinatal HIV Transmission
| Viral Risk Factors |
| Viral load Viral phenotype Viral genotype |
| Maternal Risk Factors |
| CD4+ T-lymphocyte count Advanced maternal disease Lack of ARV therapy ARV resistance Vitamin A deficiency STIs/other co-infections |
| Behavioral Risk Factors |
| Smoking Substance abuse Sexual behavior |
| Obstetrical Risk Factors |
| Vaginal delivery Preterm delivery Prolonged rupture of membranes Placental disruption/abruption, chorio-amnionitis Invasive fetal monitoring Episiotomy/forceps |
| Neonatal Risk Factors |
| Breast milk exposure Prematurity Oral thrush Gender |
High viral load is the most consistently correlated factor with risk of transmission.78–80 Other viral factors have been associated with increased risk of transmission, although less so than viral load, including viral phenotype,81–84 and viral genotype.85–87 It has been suggested that there may be different patterns of viral strain transmission at different times, with antepartum transmission more likely to be associated with the predominant maternal viral variant, while intrapartum transmission may be associated with minor viral variants.88
The role of other maternal or obstetrical factors is most obvious in the absence of antiretroviral therapy, demonstrating the importance of high viral load as a predictor of transmission. These include advanced maternal HIV infection as determined by clinical diagnosis of AIDS, low CD4+ T-lymphocyte count or percentage, longer duration of ruptured membranes before delivery, placental inflammation, and sexuallytransmitted diseases.42,73–75,89–101
Other maternal factors which have been implicated with an increased risk of transmission include preterm birth, maternal illicit drug use, vitamin A deficiency, female gender of the infant.42,73,95,96,102–107 A Malawian study has suggested that microtransfusions across the placenta may play a role in increased transmission with vaginal delivery.108
Mode of Delivery and Transmission
Cesarean section has been shown in several studies to reduce the risk of transmission of HIV.109 An individual patient data meta-analysis including data from 15 prospective cohorts demonstrated a significantly lower infection rate among infants delivered by scheduled cesarean section compared to other modes (emergency cesarean or vaginal delivery), with an unadjusted odds ratio (OR) of 0.45 (95% CI, 0.35–0.58) and an OR of 0.43 (95% CI, 0.33–0.56) adjusted for azidothymidine (AZT) use, maternal disease stage, and birth weight.110 Among more than 1400 women who received AZT, most on the PACTG 076 schedule, the rate of transmission was 2% with scheduled cesarean delivery and 7% with other modes of delivery. An international randomized trial produced similar results, with a transmission rate of 1.8% among women assigned to scheduled cesarean delivery and 10.5% in those assigned to deliver vaginally (OR, 0.2, 95% CI, 0.1–0.6).77 In an analysis stratified by AZT use, the transmission rate was 4% with scheduled cesarean delivery and 20% with vaginal delivery among those not on antiretroviral agents (adjusted OR, 0.2, 95% CI, 0–0.8), 1% with scheduled cesarean delivery, and 4% with vaginal delivery for those receiving AZT (adjusted OR 0.2, 95% CI, 0–1.7). The magnitude of the reduced transmission was similar in the two groups; but because of the small number of transmissions (six in total) in the AZT group, the difference was not statistically significant. In both studies the risk of transmission was not reduced by cesarean section performed after labor or if rupture of membranes had occurred. Neither of these studies included HIV RNA determinations or women on HAART, making it impossible to determine the potential benefit of scheduled cesarean section among women with undetectable HIV RNA or on combination therapy.
Most of the trials that demonstrate the protective effect of elective cesarean section were performed before combination antiretroviral therapy was in widespread use in developed countries. There is less evidence that cesarean section provides additional reduction in MTCT in women receiving highly active combination antiretroviral regimens who have low or undetectable maternal HIV-1 RNA levels and there does not appear to be a threshold of HIV RNA levels below which transmission does not occur. However, it has been suggested that vaginal delivery could be considered in mothers with very low or undetectable viral load. In a meta-analysis of seven prospective studies from the US and Europe there were 44 transmissions in 1020 deliveries where maternal plasma viral load was <1000 HIV RNA copies/mL at or around delivery.111 The transmission rate for mothers on antiretroviral treatment was 1% compared to 9.8% for those on no treatment. Transmission was lower with those receiving antiretrovirals and cesarean section suggesting a protective effect of both interventions, even at very low viral loads. A cost-effectiveness analysis suggests that the MTCT rate would have to be less than 0.5% before cesarean section would be no longer cost-effective.112 However, reported transmission rates in women on HAART with low undetectable viral loads have shown transmission rates of 0–3%113 and elective cesarean section may not provide additional reductions in transmission. In Europe, data suggest that cesarean section may still have an additional beneficial role, even where suppressive antiretroviral therapy is available.114,115 For those women who have access to HAART, a decision on mode of delivery should include consideration of the viral load level and clinical status. The American College of Obstetrics and Gynecology has suggested that elective cesarean section should be recommended at viral loads of >1000 copies/mL.116 Based on current data, guidelines recommend discussing scheduled cesarean delivery with all pregnant women, emphasizing the potential benefits for women with HIV RNA levels above 1000 copies/mL and indicating the lesser probability of benefit for women with lower HIV RNA levels.8
Other Obstetric Factors and Transmission
Increased risks of transmission have been described with some obstetric factors, although the magnitude of risk is much less than that associated with high viral load or mode of delivery. The relative importance of these factors is likely to be much diminished where effective antiretroviral therapy is in place. The risk of MTCT is associated with the duration of rupture of membranes during labor. In a study of 505 HIV-infected women, in those with membranes that ruptured more than 4 h before delivery, the rate of transmission of HIV-1 to the infants was 25%, as compared with 14% among mothers with membranes that ruptured 4 h or less before delivery.75 Women with low CD4+ levels were significantly more likely to transmit HIV to their offspring if the duration of rupture of membranes was 4 h or beyond.74 In a meta-analysis of 4 721 deliveries with duration of ruptured membranes before 24 h, the risk of transmission increased ∼2% for every hour of rupture of membranes (ROM) up to 24 h. In women with an AIDS diagnosis the risk of transmission increased from 8% after 2 h of ROM to 31% after 24 h.117 As artificial ROM has little obstetric benefit in normal labor, it should not be performed routinely in known HIV-positive women or areas of high HIV prevalence. Use of artificial ROM should be reserved for cases where there is fetal distress or delay in progress of labor.118,119
Some invasive obstetric investigations have been associated with higher risk of transmission. Penetrating or spiral scalp electrodes and the use of fetal scalp blood sampling could potentially create a portal of entry for the virus. There is little evidence that this is a significant risk factor, with few studies from high prevalence areas, where these procedures are uncommon, and little confirmation in cohorts who also have access to antiretroviral prophylaxis. In the European Collaborative Study in 19 European centers between 1984 and 1991, prior to widespread use of antiretrovirals, MTCT was higher with vaginal deliveries in which scalp electrodes, forceps, or vacuum extractors were used.78 Viscarello et al showed no difference in transmission rates in cases where electrodes were used or not used.120 Most current recommendations suggest the avoidance of any procedure that breaks the infant’s skin, unless unavoidable for the health of the baby.
Episiotomy may increase the exposure of the infant to HIV during delivery and increase the risk of MTCT. In the European Collaborative Study between 1941 and 1991, MTCT was higher with vaginal deliveries in which episiotomy was practiced, but only in centers where these procedures were not routine.78 In another, nonrandomized prospective cohort study among 63 HIV-positive pregnant women and their 68 infants, mothers with events involving fetal exposure to maternal blood were more likely to transmit infection.121 Even in the presence of zidovudine therapy, episiotomy was associated with a higher rate of HIV RNA positive oropharyngeal and gastric aspirates in a French study.122
A Comprehensive Approach to Preventing MTCT
The World Health Organization has promoted a comprehensive approach to preventing MTCT. This includes four components: (1) primary prevention of HIV infection; (2) prevention of unintended pregnancies among HIV-infected women; (3) prevention of HIV transmission from HIV-infected mothers to their infants; and (4) care, treatment and support for HIV-infected mothers, their children and families.123 All four of these have a role to play in preventing MTCT and optimizing care of HIV-positive women and their children. Counseling, testing and the identification of HIV-positive women is essential to provide the most appropriate care; CD4+ cell counts form a critical link between antenatal care and antiretroviral therapy (ART) services, especially for asymptomatic women. To ensure that all pregnant women who require ART are identified, efforts should be made to include CD4+ cell count measurement in the essential package of care for pregnant women.
HIV TESTING DURING PREGNANCY
Prenatal care provides an opportunity to counsel women about HIV risk and offer HIV testing. In the early years of the HIV epidemic in the United States and other countries, HIV testing was offered only to those women perceived to be “at risk” of infection. With increasing rates of infection in women and improved interventions to reduce the risk of MTCT, came recommendations that counseling about HIV infection and the benefits of testing should be “routine” in pregnancy. As early as 1995, guidelines from the CDC and leading professional associations recommended routine testing,124,125 but uptake of testing and provision of antiretrovirals for PMTCT remained less than optimal. A review of HIV testing in pregnancy from 1994 to 1999 showed a small increase in the percentage of pregnant women tested from 41% in 1995 to 52% in 1997 and to only 60% by 1998.126
This relatively low uptake using an “opt-in” approach, requiring specific informed consent and request for testing, has prompted inclusion in guidelines of an “opt-out” universal approach where all pregnant women are informed that HIV testing will be performed in the routine prenatal tests, unless she declines testing.127 A CDC review of testing strategies in 2002 showed uptake of testing in 85–98% using an opt-out approach compared to 25–83% using opt-in.128 An opt-out approach was recommended by the US Institute of Medicine in 1999, endorsed by the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP)129 and included in 2003 CDC recommendations.130 The ACOG issued guidelines in 2004 which include the use of an opt-out prenatal HIV testing approach where legally possible.131 The ACOG also recommended a repeat offer of HIV testing in the third trimester to women in areas with high HIV prevalence, those known to be at high risk for HIV infection, and women who had declined testing earlier in pregnancy. The success of “opt-out” testing may depend on the attitude and commitment of the health workers offering the test and the community norms.132,133 Data from a national survey in the US in 2002 show that 69% of women had been tested in pregnancy, but that testing rates were higher with higher income levels and where women reported some perceived HIV risk.134
Increased access to HIV testing and the changing epidemiology of the epidemic are increasing the number of women who know their HIV infection status before pregnancy. This is as high as 60–80% of HIV-positive women in the US and UK8,3 and 38.5% in Argentina.135 This also applies in some African countries where testing services have been in place for several years. In high HIV-prevalence, low-resource areas in Africa and Asia, the acceptance of HIV testing in pregnancy is high in many settings,136,137 although other program challenges remain, including fear of recognition by hospital staff and difficulties in delivering results.138,139 In other places, low uptake of testing has been a barrier to the successful implementation of some PMTCT programs.140–142 In Botswana, uptake of HIV testing by pregnant women in the PMTCT program was initially low, estimated at only 21% of antenatal clinic attendees in 2001,143 but the introduction of opt-out testing in 2004 increased testing to rates of over 90%.144 Similar results have been reported in Cameroon145 and other areas, and programs are increasingly moving to a routine, “opt-out” testing approach in both developed and developing countries.146–148
Women seen for the first time in late pregnancy or in labor are likely to have convergent risk factors, such as drug use or poor social circumstances, which also put them at risk of HIV infection. In some US settings women presenting in labor without a previous HIV test are two to four times more likely to be HIV-infected than women attending prenatal care.149–151 In developing world settings, there may be less access to prenatal care and women may only attend health facilities late in pregnancy or to deliver.119 Diagnosis during labor or early postpartum allows for interventions to reduce MTCT.152–155
Laboratory-based HIV tests may take too long to provide a result for women in labor and the use of rapid, on-site HIV tests allows for diagnosis in labor and implementation of antiretroviral prophylaxis.156–159 Successful implementation of on-site rapid testing in labor has been documented in several settings,160–162 and shown to be acceptable to most women in a large US study.163 This study, The Mother Infant Rapid Intervention at Delivery (MIRIAD) assessed acceptability and feasibility of rapid testing in labor in six US hospitals. In these settings, 8% of women had undocumented HIV status and were eligible for rapid testing in labor, and 84% of the almost 6000 women offered testing consented to this. The 2004 ACOG guidelines recommend rapid HIV testing for women in labor with undocumented HIV status; and if a rapid HIV test result is positive, the initiation of antiretroviral prophylaxis for PMTCT (with consent) without waiting for the results of a confirmatory test.131 Where staffing or infrastructure makes testing difficult in labor, early postpartum diagnosis and access to antiretroviral treatment for the infant is another option.154,164
MANAGEMENT OF THE HIV-POSITIVE PREGNANT WOMAN
Preconception Care for HIV-Infected Women
With increased access to HIV testing, increased access to ART, longer life expectancy and decreased risks of MTCT, more HIV-positive women are likely to know their HIV status before pregnancy.3,8,165 For these women, preconception care should incorporate discussion of the stage of their HIV infection, including immune status, CD4+ count and viral load, and impact of any treatment regimen. Intensive education may be required and may best be delivered by a multidisciplinary team.166 Pregnancy planning should take into account and discuss the risks of MTCT, interventions to reduce these risks, and the future care of the child. Contraception may be advised for a period of time to achieve the best possible maternal health status before pregnancy, and medical immunizations (e.g., hepatitis B, pneumococcal, or influenza) may be given if indicated.
ART may be started, or regimens adapted to be ‘pregnancy-friendly’, with the aim being maximal suppression of viral replication with agents that are also effective in reducing MTCT. Antiretrovirals with potential reproductive toxicity should be avoided where possible (e.g., efavirenz). Preconception care should also initiate appropriate prophylaxis for opportunistic infections, optimize maternal nutritional status, and screen for maternal psychological disorders or substance abuse.8 Access for HIV-infected women to assisted reproduction technology is supported by many experts in the field, although it may be limited by local regulations or service constraints,167–169 but successful interventions have been described in HIV-infected women in Europe and elsewhere.170,171 In some cases, the male partner of an infected woman will not be HIV-infected, and counseling needs to focus on the risk of infection to the partner and possible ways to achieve pregnancy without increasing this risk.166,172 Where a male partner is HIV-infected with a negative female partner, the use of sperm washing173,168 and intracytoplasmic sperm injection (ICSI) have been reported to be successful in achieving pregnancy without exposing the HIV-negative female partner to infection.36,174,175
Medical Examination and Investigations
The HIV-positive pregnant woman should undergo a thorough history and physical examination to document baseline findings and to allow early identification and treatment of any complications (Table 35-2). Tuberculosis is a common opportunistic infection in HIV-positive pregnant women in many resource-poor areas and should be looked for in these settings or in recent immigrants in developed countries.176 Depending on local policy, all HIV-positive women should be tested for tuberculosis with a skin test using intermediate strength (5-TU) purified protein derivative (PPD), unless this has been done within the previous year.
Table 35-2 Evaluation and Monitoring for the Pregnant Woman With HIV Infection
| Initial Visit | Follow-up Visits | |
|---|---|---|
| History | ||
| HIV history | Date of diagnosis | |
| HIV signs and symptoms with special attention to history of seroconversion illness or symptoms suggesting AIDS | Every visit | |
| Lowest and current CD4+ T-lymphocyte cell count; highest and current HIV viral load | ||
| History of opportunistic infections or malignancies, or hospitalizations | Every visit | |
| History of genital herpes (HSV-2) | ||
| ARV history | ARV treatment history, if any: including regimen efficacy, length of treatment, and drug switches | Every visit |
| Signs and symptoms of possible toxicity and side-effects: nausea/vomiting, abdominal pain, jaundice, extreme fatigue, skin rash. Some symptoms may be similar to pregnancy symptoms and result in delay in appropriate diagnosis and management | ||
| Adherence to regimens | Every visit | |
| ARV resistance testing if performed | ||
| Obstetric history | Number of previous pregnancies; complications and outcomes | |
| Mode of delivery of previous babies | ||
| History of genetic disorders | ||
| Use of ARV prophylaxis during previous pregnancies | ||
| Previous liveborn children and their testing history and HIV status | ||
| Planned or unplanned pregnancy | ||
| Current pregnancy | Prior contraceptive methods used, if any | |
| Last menstrual period (LMP) | ||
| Gestational age | Every visit | |
| Estimated date of delivery | ||
| Signs or symptoms of pregnancy complications: severe nausea and vomiting, elevated blood pressure, headache, significant edema, gastrointestinal or genitourinary symptoms, persistent abdominal or back pain, vaginal discharge or bleeding, fetal movement | Every visit | |
| Screen for intimate partner violence | Every visit | |
| Physical Examination | ||
| General | Vital signs, blood pressure and weight | Every visit |
| Fundoscopy, breast exam | Every visit | |
| Gynecologic examination | Examination for perineal or vaginal lesions (condyloma, ulcerative lesions, vaginal discharge), pelvic examination, checking for cervical lesions, discharge or bleeding | |
| STI screening | ||
| Obstetric examination | Fundal height, correlating with gestational age (concordant between 18 and 30 weeks) | Every visit |
| Fetal heart beat and rate, (from 16 and 19 weeks with DeLee fetal stethoscope, earlier with Doppler devices) | Every visit | |
| Fetal movements and position in third trimester | Every visit | |
| Ultrasonography | Baseline scan at 18–20 weeks for confirmation of dates and detection of anomalies. | Consider follow-up ultrasonography for growth and fluid volume at 32–36 weeks |
Adapted from Watts DH, Minkoff H. 2002;435 Anderson,38 and AIDS Education and Training Centers.223
HIV-positive women are also likely to be at higher risk of other sexually transmitted infections (STIs), and, where indicated, tests for other STIs, such as Neisseria gonorrhoeae and Chlamydia trachomatis should be performed and the baseline antibody level for T. gondii should be determined if not previously performed. Hepatitis screening may be a routine investigation in pregnancy. Where this is not the case, hepatitis B (HBV) testing should be offered to women from high prevalence areas and hepatitis C (HCV) screening considered where intravenous drug use is common.3,177
Prenatal Diagnosis
Prenatal care should include a history and assessment of risk factors for familial or genetic conditions, screening for infectious diseases that may affect the fetus and folic acid supplementation. Screening for Down syndrome, neural tube defects and other chromosomal abnormalities should follow local guidelines but may present a more complex situation. A “triple test” screening combination of α-Fetoprotein, estriol, and human chorionic gonadotropin on maternal serum at 16–20 weeks gestation will detect most neural tube defects and over half the cases of Down syndrome.178 High level ultrasound scans including assessment of nuchal translucency and anatomic ultrasound adds to the accuracy of detection. In HIV-positive women, the benefits of such testing needs to be discussed and considered carefully. One small study has demonstrated lower α-Fetoprotein in HIV-positive women on protease inhibitor (PI) treatment than in those not on PIs.179 In the 5% of women with positive serum screening tests, some results will be explained by multiple pregnancy, or ultrasound diagnosis of a birth defect. For the others an invasive amniocentesis will be required to make a definitive diagnosis. There are concerns about the potential transmission of HIV to the fetus with amniocentesis, although the magnitude of the risk has not been clearly defined, especially with effective antiretroviral treatment in place. Reports prior to the widespread use of antiretrovirals suggested an increased risk of HIV infection, especially with late amniocentesis.101,180 The risk is likely to be considerably reduced with the use of effective antiretroviral therapy.181 A multicenter Italian study of HIV-infected women who underwent amniocentesis, chorionic villus sampling or cordocentesis during the second trimester of pregnancy and delivered after 1 Jan 1997 showed no significant difference in HIV-infected infants than women who had not had invasive diagnostic procedures.182 In a Spanish program offering a combined serology screen and nuchal translucency scan assessment, 11% of 116 women screened positive, and amniocentesis undertaken in 10 women confirmed chromosomal anomalies in two.183 No MTCT was shown in this small group.
Discussion about screening tests for fetal diagnosis for HIV-infected women should include discussion about the risks and benefits of amniocentesis and screening tests should be very carefully considered if a woman does not wish to proceed to an invasive confirmatory test if indicated, as positive screening tests in the absence of confirmatory evidence can cause significant distress. If an amniocentesis is indicated and desired by the woman, it should be performed after optimization of maternal ART, or under prophylactic cover with appropriate antiretrovirals and under direct ultrasound guidance to avoid inserting the needle through the placenta or fetal injury.184
Psychosocial Issues
The successful use of PMTCT interventions depends on their acceptance by pregnant women. Where the diagnosis of HIV infection is first made in pregnancy, there are challenges in helping women work through the impact of the diagnosis and then the immediate need for PMTCT prophylactic therapy.9 An HIV diagnosis is, in itself, a traumatic event for any woman, made worse by her concerns for her unborn child, fear of transmission and guilt about putting her child at risk and sometimes a profound fear of disclosure to her sexual partner. Women with HIV may have coexistent social problems,185,186 including gender-based violence187–189 or be recent immigrants to the area with little family support available.190,191 Some women, especially in high prevalence areas where awareness of HIV is widespread may be concerned that their participation in a PMTCT program may “out” them within their community, or lead to what has been called “disclosure by association”.9 In developing countries disclosure following an HIV diagnosis in pregnancy is often very low, based on women’s fear of partner violence,192 abandonment and stigmatisation in review of 17 studies in developing countries showed that between 3.5 and 14.6% of women reported a violent reaction from their male partner.193 Alternately, an HIV diagnosis may not be the only cause of violence, but may trigger episodes of abuse in an ongoing violent relationship, and this needs to be explored with the woman.187,194 Health providers must assess the psychosocial needs of an HIV-infected woman, including screening for depression and exposure to abuse.195,196 Peer support, from counselors or in support groups, is an important contribution to the psychosocial support needs of these women, especially where traditional pregnancy education promoting breastfeeding and favoring vaginal delivery may appear to them to be contradictory and discriminating.
Drug use is common in HIV-infected women in some settings, both in developed countries and in parts of Asia, with 40% or more of women reporting drug use in some US cohorts.105,197–199 The combination of pregnancy, HIV infection and drug use presents additional management challenges. Women may be less likely to seek prenatal care,151 and be reluctant to discuss drug use because of fear of legal action, blame and stigmatization. Providers need to understand the complexity of the social situation, including the concomitant risk of violence and transactional sex, and be able to counsel in a professional and nonjudgmental manner to enable the woman to discuss her drug use and to find appropriate treatment interventions.9 Where possible, the management of an HIV-infected drug-using woman should be undertaken in close consultation with a provider expert in drug treatment, and an experienced pediatrician. Women who are dependent on opioids should be counseled about the risks and advised to enter a methadone maintenance program. Methadone use has not been shown to have adverse effects on pregnancy, but opiate withdrawal has been associated with preterm delivery and pregnancy loss.200 There is less experience with buprenorphine treatment for opioid dependency treatment, but adverse effects on pregnancy or on infants have not been reported to date and it may have a place in treatment in pregnancy.201–203
Most HIV-infected pregnant women will receive some level of ART, either for their own health or for PMTCT.8 While there is little interaction with labor-only dosing, there may be an impact when longer ART is provided. ART may impact on recreational drug use through use of similar metabolic pathways. PIs may inhibit the metabolism of drugs such as amphetamines, ketamine, lysergic acid diethylmide (LSD), and phencyclidine (PCP).204 Methadone is metabolized through the cytochrome P450 system and non-nucleoside reverse transcriptase inhibitors (NNRTIs) nevirapine and efavirenz may act to induce methadone metabolism and lead to withdrawal symptoms. There are some data to suggest that ritonavir and nelfinavir may have a similar effect, and PIs may induce or inhibit opiate metabolism. Toxicity, withdrawal and methadone dosages need to be monitored on an individual basis and methadone may need to be increased with the co-administration of nevirapine.205 Both cocaine and alcohol use in pregnancy can have severe adverse fetal effects and HIV-infected women with alcohol or cocaine dependency should be advised to enter into treatment programs.
Management of Pregnancy
The management of HIV-positive women in pregnancy should be undertaken by a multidisciplinary team where possible and should include appropriate HIV care and treatment and relevant obstetric care. Where facilities allow, women who are HIV positive should have high-risk obstetric care with increased surveillance for infections and preterm labor; they should undergo targeted investigations based on any specific antiretroviral agents or opportunistic infection prophylaxis they are receiving and on their anticipated side-effects. The use of universal precautions for health workers should be mandatory to reduce the risk of occupational exposure.119 These should include the use of gloves to handle the baby until potentially infective blood and secretions have been wiped off.
Prenatal Care
Prenatal care of HIV-positive women should include a full history and examination as detailed in Table 35-2.
Any current signs or symptoms of HIV-related illnesses should be noted, including thrush, weight loss, gingival disease, and peripheral neuropathy or generalized lymphadenopathy. Adverse pregnancy outcomes have been associated with weight loss during pregnancy in HIV-positive women in Tanzania.206 Pregnancy related complications should be monitored, including elevated blood pressure, edema and any vaginal discharge or bleeding. Rates of pre-eclampsia have been reported to be lower in HIV-positive women not receiving antiretrovirals in the United Kingdom207 but similar in HIV-positive and HIV-negative women in South Africa and Brazil. Pre-eclampsia may be increased in women on PI containing HAART.208
Women with HIV should be monitored for signs of preterm labor. Although there is conflicting evidence from studies on the association between antiretroviral use and preterm delivery,209 women with HIV may also have multiple other factors which put them at risk of preterm or low birth weight babies. Studies from the United States and Europe have shown conflicting data on differences in birth weight or gestational age for untreated HIV-positive women and uninfected women and similar controls.92,210–214 A study of 563 HIV-infected inner-city women in Atlanta did show an increase in low birth weight (adjusted OR, 1.45; 95% CI, 1.14–1.86) and preterm delivery (adjusted OR, 1.32; 95% CI, 1.04–1.70),212 but it appears that the risk factors for adverse pregnancy outcome are similar for HIV-infected women and uninfected women215 and this emphasizes the need for appropriate prenatal care for these women.216
An increase in infant mortality is associated with prematurity in European studies and with low birth weight in both infected and uninfected, exposed African children.217,218 In developing countries, the rates of low birth weight and preterm birth appear to be increased among HIV-positive women, possibly related to nutritional differences or concurrent infections.103 Data from Europe suggest a potential beneficial effect of AZT on birth weight and gestational age but a potential increase in preterm births among women receiving long-term PI therapy.219–221
Nausea and vomiting from morning sickness are common in early pregnancy, and symptoms are usually present between 6 and 16 weeks, but can persist for longer in up to one-fifth of women, and may be worsened by ART.219,222 This may interfere with ART at this stage, increasing the risk of nonadherence with regimens or reduced drug levels. It may be possible to delay the initiation of ART until after the morning sickness has abated. Where nausea and vomiting are severe they can be treated with oral or suppository antiemetics and management of any dehydration. Drug interactions with antiretrovirals should be considered, although there are no known interactions between antiemetics and antiretrovirals.9
Where the symptoms cannot be controlled in early pregnancy, all antiretrovirals should be discontinued and restarted together once nausea and vomiting has resolved.223 Hyperemesis gravidarum is defined by intractable vomiting leading to fluid and electrolyte disturbances and nutritional deficiency usually in the first trimester. These patients may require hospitalization for fluid and electrolyte replacement and ART may need to be stopped in these cases.
Laboratory Investigations
Table 35-3 shows recommended laboratory investigations for HIV-positive pregnant women, in settings of high resources, These represent the most comprehensive levels of care, Where all of these are not available, health providers will have to adjust the investigations to fit their available resources, with the most important after the HIV test being CD4+ counts and hemoglobin, and then investigations related to antiretroviral treatment monitoring.
Table 35-3 Laboratory Evaluation and Monitoring for the Pregnant Woman With HIV Infection
| Initial Visit | Follow-up Visits | |
|---|---|---|
| Laboratory Investigations | ||
| HIV | HIV test (rapid or enzyme-linked immunosorbent assay (ELISA) confirmatory test (rapid, ELISA or Western blot according to local protocol). | |
| HIV viral load and CD4+ T-lymphocyte count (total and %) | Every 3 months (at least every trimester) or as indicated | |
| HIV resistance testing consider genotyping in acute | As indicated | |
| HIV infection, virologic failure, suboptimal viral suppression on ARV therapy, or high likelihood of exposure to resistant virus based on community prevalence or source characteristics | ||
| General | Blood group | |
| Rh antibody screen | ||
| Complete blood count (CBC) | Every 3 months or more frequently based on ARV regimen or symptoms. Consider more frequently if on zidovudine regimen. | |
| Serum chemistry, electrolytes | Every 3 months or more frequently based on ARV regimen | |
| Liver enzymes (LFTs) | Every 3 months or more frequently based on ARV regimen. Consider LFTs more frequently with nevirapine containing therapy, recent changes in ARV therapy | |
| Fasting lipid measurement: consider at baseline and 3–6 months after starting PI or NNRTI regimens | As indicated, based on initial results and risks | |
| Pap smear Urinalysis and clean catch urine culture | As indicated | |
| Other infections | Rubella antibody | |
| Varicella IgG, if history unclear | As indicated | |
| Syphilis serology | As indicated | |
| Screening gonorrhea and chlamydia | As indicated | |
| Toxoplasmosis IgG: screen at initial HIV diagnosis; repeat if CD4+ T-lymphocyte <100/mm3 and not on TMP-SMZ, or with symptoms suggestive of toxoplasmic encephalitis | As indicated | |
| Bacterial vaginosis screening: consider in women with previous preterm birth, or signs or symptoms of vaginitis | ||
| Cytomegalovirus (CMV) immunoglobulin G (IgG) if CD4+ | ||
| T-lymphocyte count <100 cells/μL or if at low-risk for CMV | ||
| Consider HSV-2 serology, if history suggests | ||
| Hepatitis serology | Hepatitis A (HAV) antibody (IgG) | |
| Hepatitis B (HBV): HBsAg, HBcAb, HBsAb | ||
| Hepatitis C (HCV) antibody | ||
| TB screening | Tuberculin skin test (PPD); (induration >5 mm is positive): obtain chest radiograph if positive | |
| Disease specific | G6PD level, especially if anemic | |
| Consider hemoglobin electrophoresis, if anemic and/or at increased risk for hemoglobinopathies | ||
| Serum screening for Tay–Sachs disease, both partners if at increased risk | ||
| Urine toxicology screen, as indicated | As indicated | |
| Special investigations | Diabetes screen: Glucose 1 h after 50 g glucola – 3 h oral GTT if abnormal; additional glucose monitoring may be indicated in women on PIs (consider 20 weeks screening and repeat at 24–28 weeks if on PIs) | As indicated |
| Lactic acidosis investigations: If signs or symptoms suggestive of possible lactic acidosis in setting of | ||
| NRTI therapy: serum lactate, electrolytes, liver enzymes; consider anion gap, CPK, amylase, lipase | ||
| Triple screen (HCG, unconjugated estriol, δ-fetoprotein): | ||
| Voluntary; requires counseling; noninvasive test to determine risk of neural tube and abdominal wall defects, Down syndrome, and trisomy 18 | ||
Adapted from Watts DH, Minkoff H. 2002;435 Anderson,38 and AIDS Education and Training Centers.223
Labor and Delivery Care
A decision on mode of delivery should be made in the prenatal period in discussion with the woman and with consideration of obstetric and HIV factors. The ACOG has suggested that elective cesarean section should be recommended at viral loads of above 1000 copies/mL.116 Based on current data, guidelines recommend discussing scheduled cesarean delivery with all pregnant women, emphasizing the potential benefits for women with HIV RNA levels above 1000 copies/mL and indicating the lower probability of benefit for women with lower HIV RNA levels.8,222 Where cesarean section is performed, whether elective or emergency in HIV-infected women, they should receive prophylactic antibiotics. If it is performed after prolonged labor or prolonged ROM, longer courses of antibiotics should be considered.224
The potential benefit of elective cesarean section in reducing the risk of MTCT, and possible complications of the operation must be considered and discussed with pregnant women when deciding about the mode of delivery. In all women, regardless of HIV status, cesarean section carries a risk of complications five to–seven times higher than vaginal delivery,225 mainly due to an increased risk of anesthetic complications and of postoperative infection. Factors that increase the risk of postoperative complications in the general population may be more common in HIV-positive women. They include low socioeconomic status, genital infections, malnutrition, smoking, and prolonged labor or membrane rupture. On the basis of the available evidence, cesarean section is associated with a slightly higher risk of postoperative complications in women with HIV compared to uninfected women and some studies have shown increased rates of complications in HIV-positive women compared to HIV-negative women, regardless of the mode of delivery.226 Most have shown increases in postpartum fever, endometritis, wound infection and pneumonia.8,227–233 Complication rates are related to the degree of immunocompromise in the mother. A South African study showed that postoperative endometritis was more common in HIV-positive than HIV-negative women, but that the severity of the condition was similar in the two groups.234 The rates of postcesarean complications are similar to those for HIV-negative women with comparable risk factors, although women with advanced HIV disease and immunocompromise appear to be at a higher risk. The additional risk of complications does not outweigh the benefit in reduction of transmission to the infant. Elective cesarean section is more likely to result in complications than vaginal delivery but less likely than emergency cesarean section performed after labor has started or membranes ruptured. Modification of clinical practice, particularly use of prophylactic antibiotics, reduces the risk of complications.
Elective Cesarean Section in Low Resource Areas
While the use of elective cesarean section has been a major factor in reducing the rates of MTCT in well-resourced settings, it is not a feasible option in many high HIV seroprevalent, less-resourced areas. In these situations, there may be some cases that merit a lowered threshold for cesarean section. These would include any pregnancies where labor is expected to be prolonged or where other obstetric complications may be associated with increased transmission risk (e.g., abruptio placentae, preterm rupture of membranes). Depending on the available facilities, this may also apply to women with previous cesarean sections or breech presentations. The potential benefit has to be balanced against the risk to the mother, which may be higher in areas where healthcare infrastructure is less. The risks of operative and postoperative complications are often higher if the woman arrives late in labor with advanced complications requiring emergency intervention, such as intrauterine infection and sepsis. Vaginal delivery following cesarean section may carry a higher risk in these settings where skilled maternity care is scarce. An increase in HIV-related cesarean sections and a subsequent increase in repeated cesarean sections due to HIV may overload the health facilities in these settings. All of this will need to be considered when discussing local cesarean section policies.224
Where cesarean section is not performed, labor and delivery care should be performed in the safest possible way to reduce any additional risk of transmission. There is no evidence that shaving of the pubic areas reduces the risk of subsequent infection, on the contrary, it may increase the risk of HIV or hepatitis transmission, and should be discouraged.224 The risk of MTCT is associated with the duration of ROM during labor. As artificial ROM has little obstetric benefit in normal labor, it should not be performed routinely in known HIV-positive women or areas of high HIV prevalence. Use of artificial ROM should be reserved for cases where there is fetal distress or delay in progress of labor.118,119 In the case of premature ROM, with or without labor, the risk of HIV transmission must be balanced with the risk of premature delivery. There is no known contraindication to the use of short-term steroids to promote fetal lung maturity in women with HIV.
If induction of labor is indicated in a woman with HIV, it presents a management dilemma. Where cesarean section is available, induction of labor may not be desirable or necessary. In other cases, the need for induction must be balanced against the potential increased risk, and, if possible, the membranes should be left intact as long as possible. There is little information about transmission risk with induction of labor, but a prolonged period of ROM would theoretically increase the risk of transmission, especially in the absence of ongoing antiretroviral treatment.101
The procedure of vaginal cleansing (vaginal lavage) with chlorhexidine solution during labor was investigated as a means of reducing the risk of MTCT. In a Malawi study it was effective in reducing the risk of MTCT where membranes were ruptured for more than 4 h, but had no overall protective effect in the study group.235 Chlorhexidine vaginal cleaning was shown to have other benefits (reduced neonatal and puerperal sepsis) without controlling for HIV status of the mother or duration of ROM.236 A study in Kenya suggested that disinfection before the membranes were ruptured might be associated with a reduction of MTCT, especially with higher concentrations of chlorhexidine.237
As penetrating or spiral scalp electrodes and the use of fetal scalp blood sampling may create a portal of entry for the virus, these should be used with caution and avoided unless necessary for the health of the baby. Episiotomy may increase the exposure of the infant to HIV during delivery and increase the risk of transmission. Routine episiotomy is not recommended, and it should be reserved for those cases with a clear obstetric indication. If assisted delivery by forceps or vacuum extraction is required, there is a theoretical possibility of increasing the risk of MTCT through damage to the baby’s skin. Although some studies have shown an increased risk of infection with forceps deliveries or vacuum extraction, the evidence is conflicting across studies,78,238 and may be confounded by the obstetric reason for assisted delivery, which may be an independent risk factor for transmission. There is little information on the contribution of obstetric procedures to the risk of MTCT in the presence of ART. Despite this, consideration should be given to emergency cesarean section if there is fetal distress rather than vaginal obstetric intervention.222 This will not be possible in many instances, and where assisted delivery is required, soft vacuum cups may be preferable to metal cups or forceps.
Postpartum Care
The care provided to HIV-positive women during pregnancy should form part of a continuum of care and support for the women, their children and partners. This should ideally include; primary, obstetric, pediatric and HIV care; family planning services; mental health services; substance-abuse treatment where necessary; and ongoing psychosocial support for the woman, her children, and other family members.113,224
In most HIV-positive women, the postnatal course will be uncomplicated and HIV-infected women will not require special medical care. Table 35-4 lists important components of postpartum care for HIV-positive mothers. Further counseling and support may be needed and women should be warned before discharge about the signs and symptoms of infective complications. Peer counselors and supporters can be a positive influence and can help women and their families cope with postpartum stresses. Postpartum complications reported more frequently in HIV-positive women include puerperal sepsis, infected episiotomies, massive condylomata acuminata, urinary tract infections, pneumonia, fever, TB, unusual infections, and retention of urine. Infectious diseases and complications in HIV-positive women may require more aggressive antibiotic treatment.119,239
Table 35-4 Postpartum Care for the HIV-Infected Woman and her Child
| Counseling | Continuation versus discontinuation of antiretroviral therapy, depending on initial indications for therapy and maternal wishes |
| Additional support for adherence to antiretroviral medications given the demands for newborn care and loss of incentive of decreasing perinatal transmission | |
| Counseling on infant feeding options, supporting avoidance of breastfeeding if appropriate, with education on replacement feeding. | |
| Psychosocial support, including mental health or substance abuse treatment services; assistance with food, housing, transportation, and legal/advocacy services if needed | |
| Enhanced psychosocial support during determination of infant infection status | |
| Contraception | Contraceptive counseling, taking into consideration potential interactions of hormonal contraceptives and other maternal therapy |
| Clinical care | Review maternal status regarding the need for opportunistic infection prophylaxis, immunizations, |
| Consider repeat CD4+ T-lymphocyte and viral load to guide treatment decisions | |
| Other health maintenance such as mammograms, cervical cytology | |
| Infant | Determination of infant infection status; provision of antiretroviral prophylaxis of treatment as indicated |
| Provision of infant TMP-SMZ Pneumocystis carinii prophylaxis beginning at 4–6 weeks of age | |
| Infant immunizations as recommended | |
| Ongoing care | Referral to ongoing HIV care for mother and baby |
Adapted from Watts DH, Minkoff H. 2002;435 Anderson.38
Appropriate family planning should be discussed in the prenatal period and discussed again postpartum. Postpartum tubal ligation should be available for those who desire this.240 In low-resourced settings where prolonged breastfeeding is the norm, lactational amenorrhea is an important contraceptive method. Where women choose replacement feeding in these settings, additional contraception should be recommended. In some cultures, women may have a period of abstinence after the birth of the child, and may not wish to start contraceptive use before this ends, and should be informed how to access contraception when they do need it.
Continuity of antiretroviral treatment for those women on HAART is essential into the postpartum period and beyond. Adherence may be more difficult due to the physical changes of the postpartum period, and the stresses and demands of caring for a new baby, and additional support may be required at this time.8,241 Postpartum depression may further affect adherence in some women.
Other information in health maintenance should be given to postpartum women, including nutritional advice, advice management of common HIV-related illnesses, information about gynecological infections, such as vaginal discharge and pelvic inflammatory disease and the need to seek early treatment for these, advice on cervical screening, where available, and advice on stopping smoking or alcohol and drug use.119
ANTIRETROVIRAL TREATMENT IN PREGNANCY
Where resources permit, treatment decisions and regimen choices can be made on an individual basis and tailored to the needs of the HIV-positive woman, with all available investigations. In less-resourced areas, a more public health approach may be needed and is recommended by the WHO. In these settings, the eligibility criteria for starting HAART may be based upon clinical staging and CD4+ cell count, if available, or on WHO clinical staging alone, if CD4 counts are not available.242 Clinical staging may be more difficult in pregnant women, as assessment of weight loss may be more difficult. In this situation, health workers should take into consideration expected weight gain during pregnancy.
Antiretroviral Drug Use in Pregnancy
Although there has been increasing experience in the use of antiretrovirals in pregnant women in the last decade,243–246 there is limited long term follow-up data available for many of the antiretroviral drugs. Any use of antiretrovirals in pregnant women, whether for maternal treatment or PMTCT, needs to consider the benefits of antiretroviral treatment compared to the possible risks to the mother, fetus or neonate.247,248 Although pregnancy is not a barrier to the use of effective HAART regimens, it may necessitate consideration of the possible need to change antiretroviral dosing to fit with the physiological changes of pregnancy, the possible side-effects of the drugs in the mother, which may be worsened by or additive to pregnancy effects; and the potential teratogenic or short- or long-term side-effects of the drugs on the fetus or infant.247,249,250
On the evidence available to date, the benefits of ART during pregnancy appear to outweigh the potential side-effects or risks to mother or baby.245,251 Antiretroviral use in pregnancy can be reported to the Antiretroviral Pregnancy Registry (http://www.APRegistry.com) and treating physicians are encouraged to report to this resource. The registry is a collaborative project of the pharmaceutical industry, pediatric and obstetric providers, the Centers for Disease Control and Prevention (CDC), and the National Institutes of Health (NIH), which collects observational data on HIV-infected pregnant women on antiretroviral treatment to determine any patterns of fetal/neonatal abnormalities. All collected information is confidential and patient names are not used. The registry had accumulated data on the use of antiretrovirals in pregnancy in 4791 women by January 2005.252 Sufficient data existed in the database at this time to be reassuring that zidovudine, lamivudine, nevirapine, abacavir, and nelfinavir are not teratogenic.247,253 Similar data showing no association between congenital abnormalities and antiretroviral use has been shown in a review of 3120 pregnancies in the United Kingdom.254
Reports of the use of HAART regimens in pregnant women in resource-constrained settings have shown a reassuring safety profile. In Brazil, HAART is now the recommended regimen for pregnant HIV-positive women, with low transmission rates described: 3.57% in one study of 297 women.255 In Africa, antiretrovirals are increasingly available to pregnant women in some sites, through government programs, or donor programs such as MTCT-plus and the US President’s Emergency Plan for AIDS Relief (PEPFAR). A report from Abidjan showed a low rate of toxicity (6/182) and no transmissions in women receiving HAART.256 In Mozambique, the DREAM project has demonstrated successful use of HAART and replacement feeding with a transmission rate of 4% in infants reported at 4 weeks of age.257
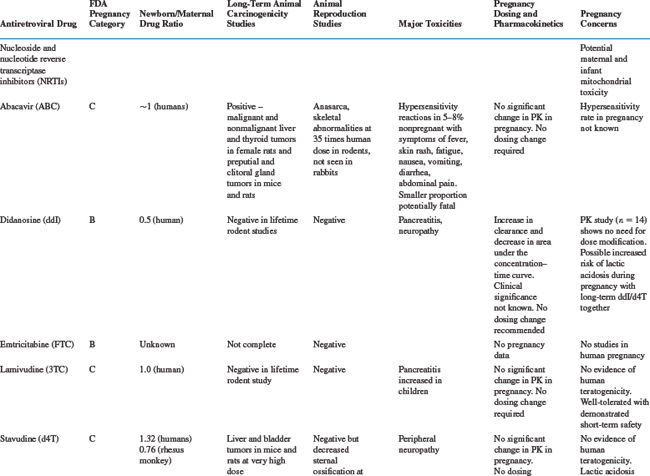
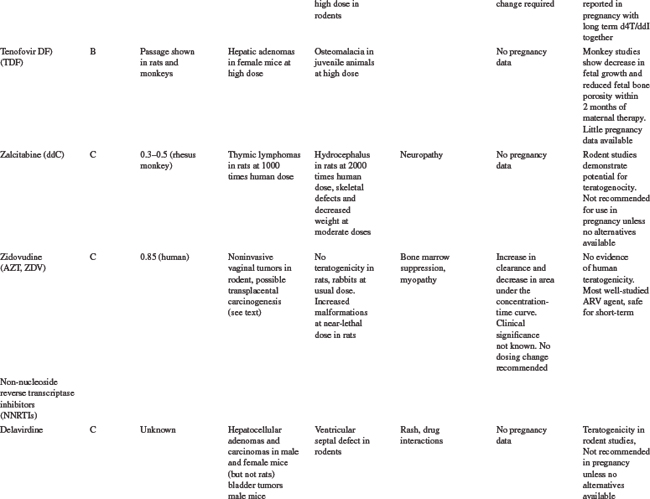
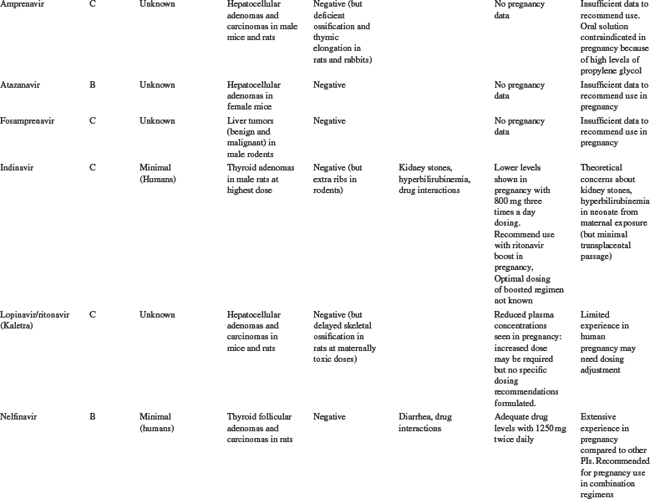
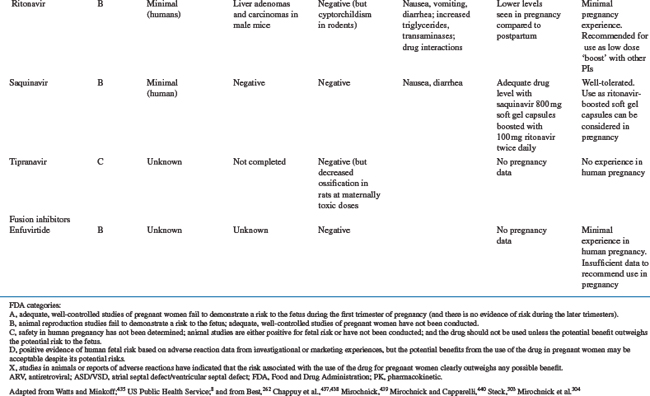
Table 35-5 summarizes the available information on FDA pregnancy risk classification, passage from mother-to-infant, animal toxicity and carcinogenicity studies, human toxicity pharmacokinetics in pregnancy and any pregnancy concerns of the currently available antiretroviral drugs. As more experience is gained with the use of newer antiretrovirals in pregnant women, this safety and toxicity information may change. Providers are encouraged to obtain the most up-to-date information as needed from resources such as the US Public Health Service guidelines258 which have a regularly updated section on antiretroviral safety in pregnancy which is available online at the AIDSinfo Web site (http://AIDSinfo.nih.gov).
Nucleoside Reverse Transcriptase Inhibitors
Zidovudine was the first antiretroviral drug shown to be efficacious in reducing the risk of MTCT and has remained one of the drugs of choice for use in pregnancy.8,259 As more information on the safety of zidovudine and lamivudine (3TC) has been accumulated than with any of the other antiretroviral drugs, they should be included in pregnancy treatment regimens where possible. The main toxicity concern with AZT is the development of anemia and neutropenia and other NRTI drugs should be considered, rather than AZT, for pregnant women with severe hemoglobin levels less than 7 gm/dL. Where anemia develops in a woman on AZT therapy, it is usually associated with macrocytosis. If resources allow, treatment of the anemia, including the use of erythropoietin, can be considered, rather than stopping the drug.38
Other NRTI drugs which can be used in pregnancy include stavudine (d4T) and abacavir (ABC) and pharmacokinetic studies have shown that standard doses are adequate in pregnancy for these drugs.258,260–262 There is limited data on emtricitabine (FTC) use in pregnancy, although animal toxicity data is reassuring and it is similar in structure to 3TC and is unlikely to need dosing changes. Didanosine (ddI) is not generally recommended for first line antiretroviral regimens in pregnancy, although it may be a component of second line regimens. Although animal studies have shown no risk of teratogenesis, congenital abnormalities have been described in 6.3% (13/205) of cases with first trimester exposure to ddI reported to the Antiretroviral Pregnancy Registry compared to a rate of 1.1% (2/190) among those with exposures later in pregnancy. No pattern of defects has been demonstrated and this will require further close monitoring.252
The antiretrovirals d4T and ddI should not be used together in pregnant women, unless the potential benefits outweigh the risks, due to an increased risk of lactic acidosis8 with prolonged therapy, although short-term combination use appears to be safe.263 Lactic acidosis is a relatively rare but potentially life-threatening condition, in women taking nucleoside reverse transcriptase inhibitors (NRTIs).251,264,265 Although all NRTI drugs may cause lactic acidosis, it is more associated with ddI and d4T than AZT, 3TC, or ABC because of a greater potential for interfering with mitochondrial replication.38 The symptoms may be nonspecific, including nausea, malaise, breathlessness, abdominal pain or discomfort, and as such, may be difficult to distinguish from pregnancy symptoms. Metabolic acidosis with elevated serum lactate and liver enzymes is common. The more widespread use of d4T as first-line antiretroviral treatment regimens in a developing countries, has been associated with higher rates of lactic acidosis than previously described in the US or Europe, and appears to be more common in women in these settings.266 There is insufficient evidence to determine whether pregnancy increases the risk of lactic acidosis on these drugs, but symptoms of pregnancy or of other pregnancy complications maybe similar to the nonspecific symptoms of lactic acidosis. Attending practitioners need to bear this in mind and monitor carefully, including careful monitoring of electrolytes, liver function tests and possible lactate levels in the third trimester.223 The symptoms of lactic acidosis generally improve with drug discontinuation.
There have been some reports of a possible association between NRTI-containing antiretroviral regimens and the development of mitochondrial toxicity in the newborn and mother, although the full consequences of this are not known.267–269 There are conflicting data across studies, although the risk of severe toxicity appears to be small. Reports in a large French cohort identified 12 cases of mitochondrial dysfunction in an 18-month prospective follow-up of 4392 NRTI-exposed children.268 In children, mitochondrial toxicity may manifest as neurologic symptoms, including febrile seizures, with or without abnormal magnetic resonance imaging or significant hyperlactatemia.270–272 In contrast, a retrospective review of more than 16 000 HIV-exposed, uninfected infants in the United States between 1986 and 1999 showed no deaths suggestive of mitochondrial dysfunction.273 Similarly, there was no increase in neurologic symptoms in NRTI-exposed versus placebo-exposed infants in the PETRA study in Africa,155 or in a large cohort in the European Collaborative Study.274 In mothers, mitochondrial toxicity may be linked to myopathy, neuropathy, cardiomyopathy, pancreatitis, hepatic steatosis and lactic acidosis.8,275
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree