T-cell prolymphocytic leukemia
T-cell large granular lymphocytic leukemia
Chronic lymphoproliferative disorder of NK cell
Aggressive NK leukemia
Systemic EBV-positive T-cell lymphoproliferative disorder of childhood
Hydroa vaccineforme-like lymphoma
Adult T-cell leukemia/lymphoma
Extra-nodal NK/T-cell lymphoma, nasal type
Enteropathy associated T-cell lymphoma
Hepatosplenic T-cell lymphoma
Subcutaneous panniculitis-like T-cell lymphoma
Mycosis Fungoides
Sezary Syndrome
Primary cutaneous CD30-positive T-cell lymphoproliferative disorders
Lymphomatoid papulosis
Primary cutaneous anaplastic large cell lymphoma
Primary cutaneous γδ T-cell lymphoma
Primary cutaneous CD-8 positive aggressive epidermotropic cytotoxic T-cell lymphoma
Primary cutaneous CD-4 positive small/medium T-cell lymphoma
Peripheral T-cell lymphoma, NOS
Angioimmunoblastic T-cell lymphoma
Anaplastic large cell lymphoma, ALK positive
Anaplastic large-cell lymphoma, ALK negative
This review will focus on the most common nodal entities, as the management of less common PTCLs can be quite different.
Prognosis of PTCLs differs by subtype. Based on data from the international T-cell lymphoma project (IPTCL) and the British Columbia Cancer Agency (BCCA), with most patients receiving an upfront anthracycline-containing chemotherapy regimen, the 5-year overall survival (OS) of PTCL-NOS was 32–35 % and the 5-year failure-free survival (FFS) was 20–29 %. AITL has a similar 5-year OS, but FFS was worse at 13–18 %. ALCL fared better, particularly the ALK+ subtype with a 5-year OS of 58–70 % and FFS of 28–60 % [8].
3 Clinical Presentation and Diagnosis
Among patients with PTCL, 38 % present with nodal disease alone, 49 % with both nodal and extra-nodal disease, and 13 % with extra-nodal disease only (commonly skin and gastrointestinal tract). The bone marrow is involved in one-fifth of patients. Approximately half of patients present with stage IV disease, and one-third have B-symptoms. Eosinophilia and pruritus is often seen, and hemophagocytic syndrome may be present [7, 9, 10].
Although pathologists can accurately distinguish B cell from T-cell lymphomas, elucidation of subtypes has been difficult due to molecular heterogeneity. T-cell antigens (CD2, CD3, CD4, CD5, CD7, and CD52) are variably expressed, and clonal T-cell receptor (TCR) gene rearrangements are characteristic but not always seen due to relative genomic stability of the TCR genes [11]. Gene expression profiling (GEP) has helped in the differential diagnosis of some subtypes; for example, AITL has been shown to have follicular T-lymphocyte derivation, and PTCL-NOS seems to be derived from activated rather than resting T cells [12, 13].
4 Management of Peripheral T-Cell Lymphomas
4.1 Frontline Therapy of Aggressive PTCL
Despite advances in the understanding of the biology of PTCLs, the standard first-line or induction chemotherapy regimens for these disorders have been derived from the treatment of B-cell neoplasms. Cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP), mainstay backbone chemotherapy for aggressive B-cell neoplasms, is the regimen typically used.
In a single-institution study with 208 PTCL patients who received frontline CHOP, complete remission (CR) was obtained in 57 %, 5-year OS was 28.5 % (22.3–36.3), and 5-year event-free survival (EFS) was 18.4 % (13.4–25.3) [14]. In general, outcomes with CHOP are inferior in PTCL compared to aggressive B-cell lymphomas [15], with the exception of ALK + ALCL. In a study by Akagi et al. [16], CR rate was 39 % in PTCL versus 67 % in DLBCL (P < 0.008), and 3-year OS was 26 % versus 50 %, respectively (P = 0.005). However, patients with ALK + ALCL patients have acceptable overall survival rates (58–70 %) with a frontline anthracycline-based regimen like CHOP, compared to ALK-ALCL (49 %), AITL or, PTCL-NOS (32–35 %) [17]. An attempt to improve these outcomes was made by adding etoposide to CHOP, with encouraging results. In an important analysis of a series of studies by the German high-grade non-Hodgkin’s Lymphoma study group, a total of 343 patients were analyzed with 289 belonging to one of the four major subtypes of PTCL [18], (ALCL ALK+ = 78, ALCL ALK− = 113, PTCL-NOS = 70, and AITL = 28). These patients were given either conventional CHOP or CHOP plus etoposide (CHOEP). Three-year event-free survival (EFS) and OS were 75.8 and 89.8 % (ALK + ALCL), 45.7 and 62.1 % (ALK-ALCL), 50.0 and 67.5 % (AITL), and 41.1 and 53.9 % (PTCL-NOS), respectively, between the two groups. Of note, the ALK + ALCL patients did particularly well with CHOEP, with a significant improvement in both EFS and OS (P <0.001 for both endpoints). Patients with AITL did better than those with ALK- ALCL, but the difference was not statistically significant. Across both treatment groups, patients with international prognostic index (IPI) >1 did worse, and those with ALK + ALCL fared better. In younger patients, with normal LDH, CHOEP significantly improved 3-year EFS (75.4 % vs. 51.0 %); however, OS was not significantly affected (P = 0.176). When ALK + ALCL was excluded, the difference in EFS between CHOP and CHOEP was not significant in younger patients but showed a trend toward improvement. Older patients (>60 years of age) did worse with the addition of etoposide. More intensive regimens have been used in an attempt to improve the response rates and survival over CHOP/CHOEP, without much success. In a single-institution study from the MD Anderson Cancer Center, 135 patients with PTCL other than mycosis fungoides were treated with either CHOP (37 %) or more intensive regimens, such as hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone (HyperCVAD) or hyperCVAD/ESHAP (etoposide, methylprednisolone, cytarabine, and cisplatin) [19]. The 3-year OS was 62 % for CHOP versus 56 % for more intensive regimens. After excluding the ALK+ ALCL patients who traditionally do better, the 3-year OS was 43 % for CHOP versus 49 % for more intensive therapies. Another prospective trial comparing CHOP/ESHAP to CHOP alone with a plan for autologous transplant in first CR did not show any advantage for the more intensive regimen [20]. Etoposide, ifosfamide, cisplatin alternating with doxorubicin, bleomycin, vinblastine, and dacarbazine (VIP-reinforced ABVD; VIP-rABVD) were compared to the CHOP/21 regimen in 88 patients with PTCL by The Groupe Ouest Est d’Etude des Leucemies et Autres Maladies du Sang (GOELAMS). Two-year EFS in the two groups was nearly identical (41 % vs. 45 %) with a median overall survival of 42 months for each arm [21]. Finally, gemcitabine-based regimens have been evaluated for PTCL and have shown good response rates but no improvement in OS when compared to CHOP/CHOEP; they also appear to be associated with more toxicity [22–24].
CD52 is expressed in up to 42 % of patients with PTCL, and attempts have been made to exploit this possible therapeutic target by adding alemtuzumab, an anti-CD52 monoclonal antibody, to CHOP (CHOP-C) [25]. In a phase I/II trial, 24 patients received 8 cycles of CHOP with alemtuzumab. The CR rate was 71 %, and at a median follow-up of 16 months, median duration of response (DOR) was 11 months [26]. Serious infectious complications were observed, including febrile neutropenia, CMV and JC virus reactivation, pulmonary aspergillosis, and staphylococcal sepsis. In a larger phase II trial (41 patients), patients received CHOP or CHOEP followed by alemtuzumab consolidation (29 patients) if a clinical response was attained following CHO(E)P. CR rate was 58.5 %; EFS and OS at 3 years in the whole intent-to-treat population were 32.3 and 62.5 %, respectively, and 42.4 and 75.1 % in the patients who received alemtuzumab [27]. Two randomized phase III trials are underway (ACT-1 and ACT-2) comparing CHOP to CHOP-C.
Bortezomib, a proteasome inhibitor, has activity in relapsed PTCL. Early, small phases I and II studies with PTCL found an ORR of 62–67 % [28]. A large multicenter phase II trial with 46 patients evaluated bortezomib plus CHOP in the frontline setting; although ORR was 76 % (CR = 65 %), 3-year OS was 47 %, and PFS was 35 %, thus similar to the results achieved with CHOP alone [29].
Other studies are underway, attempting to improve response rates combining other novel agents to CHOP, or using maintenance strategies.
5 The Role of Transplantation in Frontline Therapy
5.1 Autologous Stem Cell Transplant
High-dose chemotherapy with autologous stem cell rescue (HDT/ASCR) has been investigated as consolidative therapy in PTCL given the poor outcomes with available frontline chemotherapy, particularly in non-ALK + ALCL subtypes. However, there are no prospective randomized trials comparing chemotherapy alone with chemotherapy followed by HDT/ASCR. A meta-analysis of 21 phase I/II trials showed a trend toward survival advantage for HDT/ASCR in comparison with historical controls (HR 0.81, 95 % CI 0.31–2.13). The presence of CR at transplant and good IPI scores significantly affected survival [30]. Various other retrospective and non-randomized prospective studies report PFS and OS in the range of 35–58 % and 44–65 % with HDT/ASCR following induction chemotherapy. Again, CR at transplant, favorable-risk IPI scores, and ALK + ALCL subtype were good prognostic factors [20, 31–38].
In a phase II study by the Nordic Lymphoma Group [39], 160 patients with biopsy proven PTCL (treatment-naïve) underwent induction with 6 cycles of bi-weekly CHOEP (etoposide omitted for age >60 years) followed by HDT/ASCR in responders. Most patients presented with advanced stage disease, and 72 % had IPI scores of 2 or more. One hundred and fifteen patients underwent ASCT. The 5-year overall and progression-free survival (PFS) were 51 and 44 %, respectively. Patients with ALK-ALCL fared the best, but not much better than historical controls treated with induction chemotherapy alone.
Reimer and colleagues conducted another large prospective study evaluating ASCT in first remission after induction chemotherapy with CHOP. Eighty-three patients were enrolled with 55 going on to consolidation with transplant. The 3-year OS rate was 48 % but was more favorable (71 %) in patients who underwent ASCT [37].
Randomized trials are still needed to establish HDT/ASCR as a standard frontline consolidative approach. It remains an option for patients with significant responses to induction chemotherapy.
5.2 Allogeneic Transplant
In principle, allogeneic transplant for PTCL would utilize a graft-versus-lymphoma effect (as well as infusing a lymphoma-free graft) and might perform better than HDT/ASCR [40]. However, there are no randomized, phase III trials evaluating allogeneic transplant in the frontline setting, and most of the retrospective analyses have been conducted in the relapsed/refractory setting.
A study by Kanakry et al. reviewed the outcomes of 44 consecutive, related-donor allogeneic transplants for PTCL, 24 with reduced-intensity conditioning (RIC) and 20 with myeloablative conditioning (MAC), and included 18 reduced-intensity/haploidentical transplants. The estimated 2-year PFS was 40 % and OS was 43 %, with a tendency toward superior outcomes for those undergoing transplant in first remission (P = 0.08). Of note, approximately 50 % of the patients presented with poor-risk or chemorefractory disease, and 25 % received transplant with active disease. Relapse in RIC and MAC regimens were comparable, with less treatment-related mortality (TRM) with RIC [41]. A recent phase II study evaluated frontline autologous and allogeneic transplants in different age groups with CHOP-alemtuzumab conditioning (n = 86) and found that auto/allotransplants improved DFS in younger patients only [42].
In summary, allogeneic transplant is usually recommended in the relapsed setting but can be an option in first remission in select poor-risk patients.
6 Treatment of Relapsed/Refractory Disease
Traditionally, refractory or relapsed PTCL has been treated with second-line chemotherapy regimens often followed by autologous or allogeneic transplant. However, the recent development of therapies specific to T-cell lymphomas is rapidly changing this paradigm. We will briefly discuss the role of transplant and then discuss chemotherapy and other novel therapies in this setting.
There are no large randomized trials evaluating transplant in relapsed and refractory disease, and our practices are derived mainly from retrospective data. A retrospective study by Le Gouill et al. reported on 77 patients with PTCLs (84 % ALCL, AITL, or PTCL-NOS) treated with allotransplant. PFS and OS were 53 and 57 %, respectively, with a 43 months follow-up following allogeneic transplant [40]. Many of these patients had received at least two prior lines of therapy including autologous transplant (25 %). Of note, AITL patients fared the best (OS rate 80 %) followed by PTCL-NOS and ALCL (63 and 55 %, respectively). TRM was 21 % at 100 days and 34 % at 5 years. A study by Kyriakou et al. [43] found similar PFS/OS for AITL patients, with chemotherapy-sensitive disease faring better. Similar outcomes have been seen in other retrospective analyses; however, early TRM remains a significant problem. It has been difficult to compare autologous and allogeneic transplant in the relapsed/refractory setting, but in general, results appear to be better with allogeneic transplant. However, a recent retrospective study by Smith et al. [44] suggested a possible benefit in favor of autologous transplant. Of 241 patients with PTCL, 115 underwent autologous and 126 allogeneic transplant. Patients receiving autologous versus allogeneic transplant had significantly greater adjusted 3-year OS (59 % vs. 47 %, P = 0.046) and lower NRM or non-relapse mortality (P < 0.001). In the absence of mature data from trials of novel agents, transplant following chemotherapy remains the recommended option for patients considered good candidates.
7 Non-transplant Therapies for Relapsed/Refractory PTCLS
Transplant may not always be an option for PTCL patients with relapsed/refractory disease due to presence of co-morbidities or unavailability of donors, and even when it is possible, it does not have robust outcomes. There is a clear need for the development of therapies directed specifically toward T-cell neoplasms, and the field has advanced significantly in the last few years. Once again, there is a paucity of large randomized phase III studies, so we will review mainly data from phase II studies, as well as ongoing trials.
7.1 Chemotherapy
Among the various chemotherapeutic agents that have been investigated in the relapsed setting, the nucleoside analog gemcitabine has shown significant activity. In a small study, 13 patients with PTCL-NOS and mycosis fungoides (MF) received single agent gemcitabine, and 9 of 13 patients responded [45]. The long-term outcome data for gemcitabine monotherapy in relapsed PTCL (PTCL-NOS and MF) were published in 2010 by the same group [46]. Five out of 20 patients with PTCL-NOS had continuous CR at the time of final follow-up, with a median duration of CR of 34 months (range 15–60 months).
In a small trial, 24 elderly patients received treatment with gemcitabine in combination with oxaliplatin and dexamethasone; the ORR was 25 % and the median OS was 14 months [47].
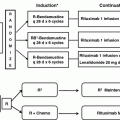
The other chemotherapeutic agent that has shown promise in the treatment of PTCLs is bendamustine. Bendamustine is a “dual-structure” drug consisting of an alkylating portion, derived from nitrogen mustard, and a purine analog portion. An open-label phase II trial of bendamustine was conducted in patients with relapsed PTCL (primarily AITL and PTCL-NOS). Patients had received a median of one previous line of therapy, thus not heavily pretreated [48]. In the intention to treat (ITT) analysis, the ORR was 50 % (CR 28 %), and the PFS and OS were 3.6 and 6.2 months, respectively. However, only 25 % of patients completed the planned six cycles of bendamustine. Common reasons for early discontinuation included toxicities (cytopenia and infections) and disease progression. Future studies will likely incorporate bendamustine into multidrug regimens.
Forodesine is a potent inhibitor of the enzyme purine nucleoside phosphorylase, which leads to intracellular accumulation of deoxyguanosine triphosphate (dGTP), causing reduced proliferation of lymphocytes and apoptosis [49]. In a phase I–II study, patients received oral forodesine. A maximum tolerated dose (MTD) was not reached, and the optimal biologic dose was determined as being 80 mg/m2 daily. Thirty-six patients received this dose and the ORR was 39 %. Long-term data are awaited.
8 Antibody-Directed Therapy
8.1 Brentuximab Vedotin
Systemic ALCL is characterized by strong CD30 positivity, and the search for an effective monoclonal antibody has been an active area of research in the field of PTCL. Brentuximab vedotin (BV) is an antibody–drug conjugate, comprising an anti-CD30 monoclonal antibody conjugated to the potent antimicrotubule agent monomethyl auristatin E (MMAE). The monoclonal antibody binds to CD30-positive neoplastic T cells and is internalized; MMAE is then cleaved from the molecule exerting its action through inhibition of microtubule formation. Hence, a potent chemotherapeutic agent can be delivered in a targeted manner [50]. A phase II multicenter study by Pro et al. evaluated the activity of BV in 58 patients with systemic ALCL who had relapsed after at least one prior line of therapy. The ORR was 86 % (CR = 57 %, PR = 29 %) [51] with a median overall response duration of 12.6 months, and CR duration of 13.2 months. Grade 3–4 adverse events included neutropenia, thrombocytopenia, and peripheral sensory neuropathy. On the basis of this study, BV was approved by the FDA for use in relapsed systemic ALCL.
A recent phase II multicenter study evaluated the efficacy and safety of brentuximab vedotin in relapsed/refractory PTCL other than ALCL. Of 34 evaluable patients (PTCL-NOS n = 22, AITL n = 13), ORR was 41 % with a median PFS of 6.7 months. Interestingly, there was no correlation between CD30 expression and response [52, 53]. A retrospective analysis of brentuximab vedotin in CD30-positive relapsed lymphomas in older patients showed a 100 % RR in systemic ALCL, with tolerable toxicities [54]. A strategy of combining standard frontline therapy (CHP) with BV was shown to be safe and effective in a phase I trial and is now being tested in an international randomized phase III trial comparing CHOP to CHP-BV as upfront therapy for CD30-positive PTCL. The results are eagerly awaited at present [55].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree