Malignant peritoneal mesothelioma (MPM) is a rare but highly lethal cancer that arises from the serosal membranes of the abdominal cavity. MPM represents about 15 to 20 percent of all malignant mesothelioma diagnoses which translates into approximately 350 to 450 new cases in the United States annually. Patients with MPM almost always succumb to disease progression within the abdominal cavity. There is a strong etiologic association with asbestos exposure although the link has not been as firmly established as in the pleural variant; in almost 40% of individuals with MPM no definite exposure to asbestos can be established. Because of its rarity, progress in the understanding of its molecular biology and advances in the development of more efficacious systemic treatments have been very limited. The disease afflicts gender equally but women and younger patients tend to have a better outcome. Systemic chemotherapy using a combination of cisplatin and pemetrexed has limited efficacy in controlling the disease long-term while surgical cytoreduction (CRS or cytoreduction surgery) with hyperthermic intraperitoneal chemotherapy (HIPEC) has become the accepted initial therapeutic intervention in appropriately selected patients with this disease.
The first description of a diffuse primary malignant process of the peritoneal serosa was made in a publication in 1908.1 In the report, a 32-year-old male miller with diffuse abdominal pain and ascites was noted to have an extensive and diffuse intraperitoneal neoplastic process that was not amenable to resection at surgical exploration. He was treated with palliative interventions and succumbed to disease 1 year later. Fifty years later, a literature review of 13 pathologically confirmed cases of what was first termed malignant peritoneal mesothelioma or MPM established this disease as a distinct pathological entity.2 Subsequently, an increased number of documented cases were reported in the medical literature and an understanding of the risk factors and clinical features of the condition began to emerge. Moertel3 published a comprehensive review of the subject in 1972 and in 1983 Antman et al4 at the Dana Farber Cancer Center published the first report detailing results of an integrated treatment strategy using a combination of operative resection, whole abdominal radiation, and systemic chemotherapy for patients with MPM. In that study, 18 previously untreated patients with MPM received a doxorubicin-containing regimen that was associated with substantial toxicity. Fourteen had measurable or evaluable disease and of these 14 patients, 6 (43%) had a measurable response. The median survival in the six responding patients was 22 months, while survival for the remaining eight patients who had stable or progressive disease was 5 months. Despite the subsequent development of systemic chemotherapy regimens consisting of pemetrexed and cisplatin or gemcitabine, MPM is still considered a chemotherapy-resistant cancer; overall response rates using these regimens are about 30% and median overall survivals (OSs) are 13 to 27 months.5,6 A single-arm phase II study of tremelimumab (anti-CTLA) in mesothelioma patients resulted in an overall response rate of only 7% and a disease control rate of 31%; however, OS in the study was only 11 months.7
Because of the recognition that patients with MPM will succumb to disease progression within the abdominal cavity, strategies designed to control disease in this region should translate into improved survival. Over the past 20 years a number of centers have reported very favorable outcomes in selected patients following CRS and HIPEC primarily using mitomycin C or cisplatin. Currently, CRS and HIPEC are considered the best first-line option for MPM patients with a good performance status and a disease burden amenable to complete resection.
A recent series from Turkey of 35 patients who presented with MPM provides insight into the natural history of this disease. Most patients were diagnosed late in their disease course and were treated with palliative interventions; the reported mean OS was 16 months (range 3 to 52 months).8 Over 80% of patients had a history of asbestos exposure and factors associated with poor survival were age greater than 60 years and decreased performance status.
The most frequent presenting symptoms are abdominal distention usually secondary to ascites and pain.4,9,10 The most common age range for patients at initial presentation is between 40 and 65 years, although the disease can be diagnosed in teenagers and the elderly.11 Genders are afflicted almost equally in MPM which is in contrast to the pleural variant which afflicts men more commonly. As the disease progresses patients experience decreased appetite, early satiety, and decreased oral intake with secondary loss of lean body mass. Bowel obstruction is a rare initial manifestation of MPM but is commonly associated with advanced disease and shortened survival.12 Intra-abdominal hemorrhage can occur but is also very rare. Notably, morbidity and mortality from MPM are almost invariably due to disease progression within the peritoneal cavity.
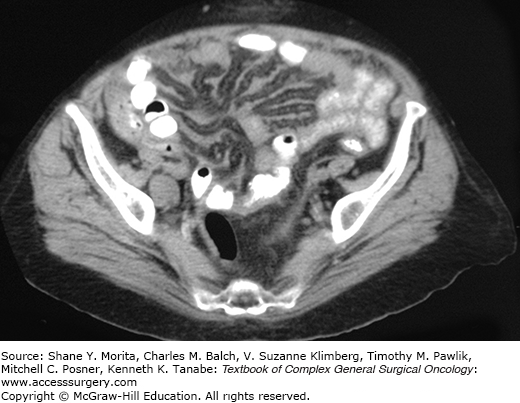
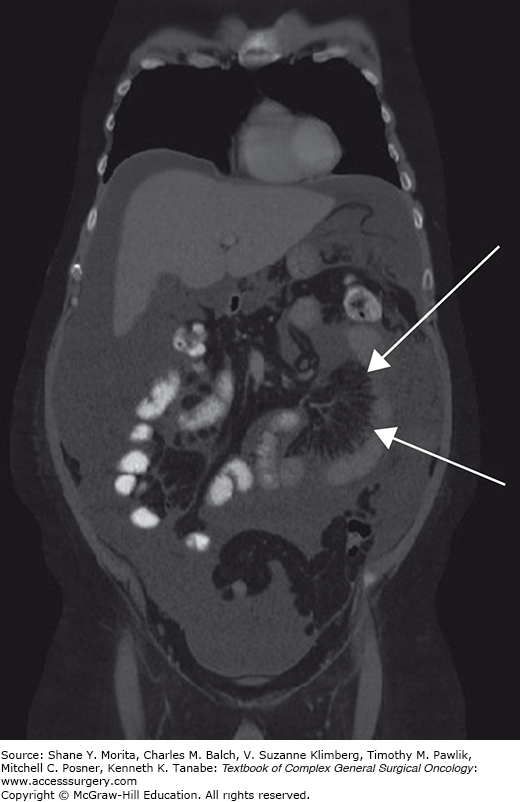
Initial imaging with ultrasound or computed tomography (CT) typically shows a diffuse process in the abdominal cavity consistent with malignancy. CT findings that are consistent with diffuse MPM (DMPM) include diffuse thickening of the peritoneum or mesentery in an irregular or nodular fashion, focal intraperitoneal masses, omental thickening or mass, and ascites.13 Specific findings on CT scan are related to an increased or decreased likelihood of encountering disease that is amenable to complete gross surgical resection.14 For example, in individuals with ascites and an omental mass but minimal findings on the visceral peritoneum (small bowel serosa and mesentery) the disease is more likely completely resectable than in those that have foreshortening of the small bowel mesentery of imageable disease around the visceral peritoneum (Figs. 119-1 and 119-2). CT findings consistent with bowel obstruction are very ominous.14 Magnetic resonance imaging (MRI) using specific acquisition protocols is being increasingly utilized.15,16 Positron emission tomography (PET) or PET/CT have inconsistent findings but has been advocated by some as useful in assessing intra-abdominal disease.17 However, it is probably best used to identify metastatic extra-abdominal disease in high-risk patients.18 If no primary pathology is present within the gastrointestinal tract then MPM should be included in the differential diagnosis. Evaluation for an occult gastrointestinal primary tumor with upper and lower endoscopy should be performed. In women a gynecologic primary neoplasm is often presumed and in some women an initial operative exploration is performed for that indication.
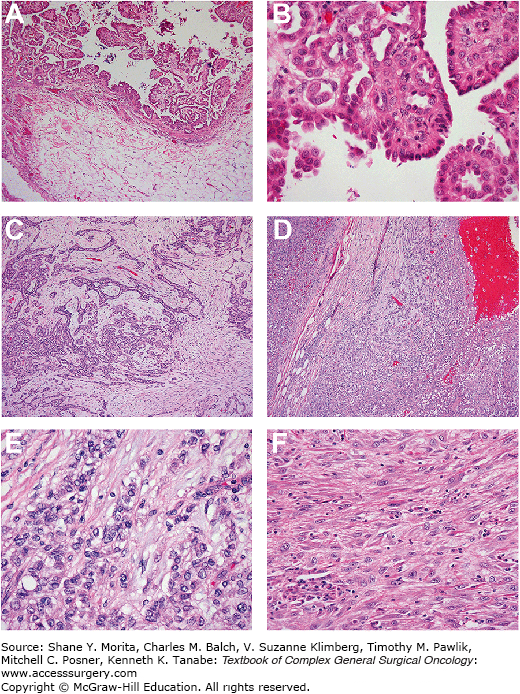
A tissue diagnosis via a core needle biopsy should be performed under CT, ultrasound guidance, or laparoscopy. Diagnostic laparoscopy has the added advantage of allowing for direct visualization of tumor burden and, consequently, the identification of patients whose disease is amenable to operative intervention.18 Abdominal paracentesis may be diagnostic; however, there are usually only scant numbers of malignant cells present in ascites for diagnosis.19 A consensus statement update from the International Mesothelioma Interest Group (IMIG) was recently published that provides guidelines for the pathologic diagnosis of malignant mesothelioma.20 There are three broad histological subtypes of MPM: epithelioid, sarcomatous, and the mixed/biphasic type; the epithelioid subtype is the most common and associated with the best prognosis (Fig. 119-3).10,21 On light microscopy cells will not have typical epithelial or glandular features seen in gastrointestinal cancers. It is generally recommended that multiple immunohistochemical markers with a sensitivity or specificity of greater than 80% be used to render a correct diagnosis.20 Antibodies that stain positive in MPM and are most commonly used are calretinin, cytokeratin 5 or 5/6; Wilms Tumor 1 (WT-1), and D2-40 (podoplanin).22 Epidermal growth factor receptor (EGFR) is also positive in 94% of MPM tumors.23 Antibodies that stain negative include CEA, B 72.3, MOC-31, and Ber-EP4.20,24 In a study of 64 MPM tumor samples the degree of tissue invasion was shown to strongly correlate with mitotic rate, nuclear grade, inversely with completeness of cytoreduction, but was not correlated to tumor burden as reflected in the peritoneal cancer index (PCI).23 In a subsequent study by the same group the degree of tissue invasion was codified as 0 (none), 1 (adjacent stroma), or 2 (deep tissue invasion). Deep tissue invasion was identified as an independent factor associated with shortened survival.24
FIGURE 119-3:
Various histologic variants of DMPM that are encountered. Panel A shows typical epithelioid malignant mesothelioma, noninvasive (without stromal invasion). There are papillary structures projecting into the peritoneal space (above) without invasion into stroma or fat (below). Panel B shows epithelioid malignant mesothelioma, tubulopapillary type. The noninvasive portion is composed of papillary projections with tubules within the cores. Panel C shows epithelioid malignant mesothelioma with stromal invasion. The invasive tumor forms discrete tubules that invade within a fibrous stroma. Panel D shows deeply invasive epithelioid malignant mesothelioma; the tumor invades fatty tissue (adipocytes seen at the bottom left). Panels E and F show features that are consistent with aggressive clinical behavior; panel E is epithelioid malignant mesothelioma with solid growth pattern. The cells are epithelioid, with rounded shape, but lack differentiation to tubular or papillary structures. Panel F is a sarcomatoid malignant mesothelioma; the tumor cells are spindled, resembling sarcoma and lacking cohesive epithelioid growth.
Well-differentiated papillary mesothelioma is a rare condition that is usually found incidentally in women undergoing a surgical procedure for an unrelated condition. Its malignant potential is not fully understood. In a series of 26 cases followed for a mean of 47 months (range 4 to 192 months) in which the lesions were excised at operation, only 1 patient had documented recurrence or progression which was also found coincident with a second laparotomy.25
As MPM presents as a diffuse intra-abdominal process, the traditional tumor-nodal-metastases (TNM) staging paradigm is not particularly relevant. However, a modified or adapted “TNM” staging system has been proposed that takes into account the peritoneal cancer index (PCI) as described by Sugarbaker26 as a surrogate for T stage, the presence of intra-abdominal lymph node metastases (N), and the absence or presence of extra-abdominal disease (M).27 T stage is calculated from the PCI (Table 119-1). Stage I includes patients with a low PCI (T1), stage II includes patients with moderate to high PCI (T2-3), and stage III includes those with any T but with lymph node or systemic metastases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree