25 Malignant Gliomas
Over 23,000 new cases of primary nervous system tumors are diagnosed in the United States every year, resulting in nearly 14,000 deaths annually.1,2 Although primary intrinsic malignant brain tumors occur less frequently than either extra-axial tumors or metastatic disease to the central nervous system (CNS), they are disproportionately lethal.3 Despite significant advances in diagnostic neuroimaging and in techniques for surgical resection and for the delivery of chemotherapy and radiation over the past 50 years in neuro-oncology, improvements in the survival of glioblastoma multiforme (GBM), the most common type of malignant glioma, remain modest. As such, innovative translational research and clinical trials of novel therapeutics continue to be actively pursued for this disease.
Gliomas are tumors of neuroepithelial origin derived from glial cells or cell precursors.4 The designation malignant refers to the abnormal proliferation, invasion, and metastatic potential of the cells forming these tumors. Glioma is a more inclusive classification encompassing two major basic histopathological subtypes: astrocytoma and oligodendroglioma. Current treatment consists of surgical resection, postoperative radiation, and chemotherapy, and has been found to provide a slight increase in overall survival.5
 Tumor Types
Tumor Types
World Health Organization Classification
Several different systems of classification of malignant gliomas based on survival, histological, and molecular data have been used. Today, the main system of classification of malignant gliomas is the World Health Organization (WHO) system.6 The system divides astrocytomas into four grades: grade I, pilocytic astrocytomas; grade II, diffuse low-grade astrocytomas; grade III, anaplastic astrocytomas; and grade IV, glioblastomas. Although grade III and IV tumors are commonly designated as the “malignant” varieties, grade II astrocytomas could also be considered as such, given the high rate of malignant degeneration and ultimate fatality of low-grade astrocytomas. The WHO system designates those tumors with nuclear atypia alone as grade II, those with higher degrees of mitosis and nuclear atypia as grade III, and reserves a grade IV designation for those exhibiting microvascular proliferation or necrosis (Fig. 25.1).
Oligodendrogliomas and oligoastrocytomas are classified by morphological appearance, with the term anaplastic added to those with a high incidence of mitoses and nuclear atypia.
Molecular Classification
Glioblastoma was one of the first tumor types studied in the large-scale National Institutes of Health (NIH) effort, The Cancer Genome Atlas (TCGA) project, which seeks to systematically and robustly characterize the molecular alterations found in cancer. Gene expression profiling of GBM found four distinct molecular subtypes of primary glioblastomas (classical, proneural, mesenchymal, and neural) that correlate with genomic abnormalities, bear prognostic significance, and may predict benefit of therapy.7
The classical GBM group is characterized by overexpression of epidermal growth factor receptor (EGFR), as well as the absence of TP53 mutations. Clinically, the classical group survived the longest of the subgroups in response to aggressive treatment.
Proneural tumors make up the largest proportion of GBMs and frequently harbor mutations of TP53, PDGFRA, and IDH1. Patients with the proneural type tended to be significantly younger than the other subgroups and survive longer, but did not appear to benefit significantly from aggressive treatment.
Mesenchymal GBMs are categorized by NF1 mutations, as well as by having frequent mutations in PTEN and TP53. Patients in the mesenchymal subgroup had the worst prognosis; although they appeared to realize a benefit from aggressive chemotherapy, the benefit not as much as that seen in the classical subgroup of GBM.
The neural group did not have significant rates of unique genetic alterations.
With an increasing number of studies validating the above classification scheme, future treatments for malignant gliomas may become more tailored to molecular genetic subgroups.
 Epidemiology
Epidemiology
Gliomas constitute 29% of all primary brain tumors, and 54% of gliomas are glioblastoma, by far the most common sub-type. The reported incidence of grade III anaplastic astrocytomas (AAs) varies widely from 10 to 30% of all the gliomas.1,3 In the pediatric population, AA and GBM constitute only a minority (< 10%) of all intracranial tumors.8
Glioblastoma (WHO grade IV) is rare in patients younger than 30 years of age, and most frequent between the ages of 45 and 65. The male-to-female ratio is 1.6:1. Anaplastic astrocytoma (WHO grade III AA) tends to occur in middle age (35 to 55 years), generally a decade before GBM appears.6 The annual incidence of malignant gliomas is approximately 5 cases per 100,000 people.3 Anaplastic oligodendroglioma (AO) commonly occurs around the fourth decade of life. The incidence of these tumors has increased slightly over the past two decades, especially in the elderly, likely as a result of improved diagnostic imaging.9
Special Consideration
• Malignant gliomas tend to spread or recur in adjacent brain regions along white matter tracts. Spread outside the central nervous system (CNS) is extremely rare.
Approximately 60% of gliomas are located in one of the cerebral hemispheres, with the frontal lobe most common (25.6%), followed by the temporal lobe (19.6%) and parietal lobe (12.6%). Glioblastoma multiforme is uncommon in the region of the third ventricle (< 1%) and rarely occurs in the posterior fossa. Although most GBMs are centered in the deep white matter, approximately 10% may present on the surface with an epicenter at the gray-white junction in the brain.3
Malignant gliomas are believed to occur spontaneously, with the majority of cases having no genetic or environmental cause identified. Genetic syndromes including neurofibromatosis types 1 and 2 and Li-Fraumeni and Turcot’s syndromes, are putatively responsible for around 5% of these tumors, and these patients often have a positive family history.10 Ionizing radiation is the only well-established risk factor, whereas the evidence for head injury, foods, occupational exposures, electromagnetic fields, and cellular telephones as causative agents is inconclusive.11,12 Aspects of the immune system may play a protective role, as patients with a history of asthma, eczema, allergies, and high levels of immunoglobulin E (IgE) have been found to have a decreased risk of gliomas.13
 Clinical Presentation
Clinical Presentation
Patients with malignant gliomas present with a variety of symptoms, depending on the size, location, and relative mass effect of their tumors. Initial symptoms most commonly include headaches, seizures, focal neurologic deficits, confusion, memory loss, and personality changes. There are no clinical findings unique to the various tumor types (anaplastic astrocytomas vs anaplastic oligodendrogliomas vs glioblastomas); however, more aggressive, rapidly progressing lesions tend to have a more rapid onset and severity of symptoms, whereas lower grade tumors have a more insidious course. In recent years, the interval from symptom onset to diagnosis is diminishing, given the increasingly widespread availability of neurodiagnostic imaging. Classically, the headache associated with mass lesions causing increased intracranial pressure is worse in the morning after awakening from sleep and decreases throughout the day. However, most patients experience headaches that are indistinguishable from other, nonmalignant causes of headaches.14 When severe, the headaches may be associated with nausea and vomiting, indicating increased intracranial pressure. Tumors located in the posterior fossa are associated with a higher incidence of obstructive hydrocephalus and can have the additional findings of ataxia, dizziness, and incoordination.1
Special Consideration
• Clinical presentation is determined mostly by anatomic location, mass effect, and growth rate, but not necessarily histology. Tumors in eloquent locations that cause mass effect, regardless of size, can present with rapid onset of symptoms, whereas very large tumors in non-eloquent areas (e.g., right frontal lobe) may be clinically silent for a long time. Similarly, seizure activity does not correlate with tumor grade, but may be related to IDH1 mutational status and correlated with 2-hydroxyglutarate production.
 Imaging Studies
Imaging Studies
The initial study that is appropriate for diagnosing a malignant glioma is usually either a head computed tomography (CT) or brain magnetic resonance imaging (MRI) scan. On CT, AAs may appear as low- or mixed-density lesions with indistinct borders that cause mass effect and edema with variable amounts of enhancement.15 A GBM typically displays more heterogeneous density and can have areas of hemorrhage, necrosis, or cysts. Oligodendrogliomas are also hypodense and often contain areas of calcification.16
Currently, MRI is the imaging modality of choice for malignant gliomas, as assessment of the size and extent of gliomas is more accurate based on MRI than on CT. On MRI, both AA and GBM display considerable heterogeneity. Most tumors of both types enhance with gadolinium contrast, but there is variability, with some studies finding that 30 to 50% of AAs do not enhance, whereas only around 5% of GBMs do not.17 The GBMs also more commonly display a ring-enhancing appearance due to areas of central necrosis as well as spread across white matter tracts, including the corpus callosum or anterior and posterior commissures. Such spread across the midline in a classic “butterfly” appearance is more common in glioblastoma.
Special Consideration
• The differential diagnoses of contrast-enhancing “butterfly” lesions crossing the corpus callosum into both hemispheres includes CNS lymphoma, oligodendroglioma, and glioblastoma. These lesions should be biopsied to avoid misdiagnosis, as CNS lymphomas and oligodendrogliomas with 1p19q deletions may be more responsive to adjuvant therapies than glioblastomas and have a significantly different prognosis.
However, the appearance of malignant gliomas on contrast-enhanced MRI does not fully represent the extent of tumor, which is often highly infiltrative. Tumor cells are often clearly seen on microscopic histopathological analysis infiltrating into brain tissue that appears as uninvolved, nonenhancing areas on MRI. Owing to this fact, surgeons often attempt a maximal safe resection into areas of T2/fluid-attenuated inversion recovery (FLAIR) signal suggestive of nonenhancing tumor. The current standard of care is for radiation oncologists to treat a 2-cm margin around the imaging-defined tumor. These strategies are validated by autopsy studies, where diffusely infiltrative cells are identified well beyond areas of contrast enhancement, often distantly into the opposite hemisphere.18
Newer imaging modalities are being increasingly used to help with neurosurgical planning and resection, as well as to define and monitor responses to treatment. When tumor resection in or near an eloquent area is planned, surgeons now use functional MRI (fMRI) and diffusion tensor imaging (DTI) to better delineate areas that can be safely removed from critical eloquent areas, such as speech and motor cortex and associated fiber tracts, where removal may lead to unacceptable neurologic deficit.19 Intraoperative MRI (iMRI) guidance is being used increasingly to aid resection.20
Pitfall
• Despite being “malignant,” 30 to 50% of anaplastic astrocytomas and 5% of glioblastomas may lack contrast enhancement on either CT or MRI, leading to a false imaging diagnosis of a low-grade glioma.
• Radiation necrosis and treatment effect (i.e., pseudoprogression) can be mistaken for true disease recurrence. Metabolic imaging studies, such as MRS and PET, can be helpful in distinguishing pseudoprogression from true progression.
Magnetic resonance spectroscopy (MRS) can help differentiate areas of tumor from necrosis or benign lesions by measuring the relative concentration of several metabolites. Malignant gliomas typically show a relative increase in choline (Cho), which is associated with cell membrane synthesis, and a decrease in N-acetyl aspartate (NAA), which is typically reflective of neurons. Other metabolites, such as lipids and lactate, are useful in differentiating entities such as abscess, radiation necrosis, and pseudoprogression from true tumor.21 Positron-emission tomography (PET) using various tracers (FDG, FLT, DOPA) is similarly being used to help diagnose tumors and shed light on their malignant potential; aid in guiding resection, stereotactic biopsy, and radiotherapy targets; as well as in monitoring response to therapy.22
 Natural History and Survival
Natural History and Survival
Prior to the last decade, progress in the treatment of malignant gliomas had remained relatively stagnant compared to successes in the treatment of other types of brain tumors, including medulloblastomas and meningiomas. More recently, data from the 2012 Surveillance Epidemiology and End Results (SEER) population analysis revealed an encouraging trend for malignant glioma patients. Patients in the years 2000 to 2008 exhibited a statistically significantly improved overall survival compared to patients diagnosed in the decades earlier.1 This suggests that new developments in diagnostics, surgical technique, and radiotherapy and chemotherapy have led to some realized benefits for patients.
Special Consideration
• With the improved overall survival of patients with malignant gliomas over the past decade, there has been a seemingly increased incidence of distant recurrences and multifocal gliomas. As better local control of malignant gliomas is achieved through more aggressive surgical resection, radiotherapy, and chemotherapy, newer systemic therapies are needed to treat the distant and multifocal CNS recurrences. The incidence of multifocal glioma is 1.5% at initial presentation, and 7.5% at recurrence.
 Prognostic Factors
Prognostic Factors
Several prognostic factors, including age, Karnofsky Performance Scale (KPS) score, extent of surgical resection, postoperative radiation treatment, degree of necrosis within the resection pathology, and the degree of enhancement on pre-operative and postoperative MRI, have been observed to influence outcome in malignant glioma patients.23–25 Patients under 40 years of age tend to survive longer than those 40 and over, independently of their improved ability to tolerate treatments such as chemotherapy and surgery.24 However, this may be due to the fact that patients under 40 are more likely to have the “proneural” subtype of GBM, which is associated with longer survival.26 The KPS is also a strong independent predictor of outcome in clinical trials, with a cutoff value of 70 stratifying patients into groups with large differences in survival.27
Pitfall
• Patient age has long been recognized as a strong negative prognostic factor for patients with malignant gliomas. However, recent studies have shown that gene expression profiling strongly predicts survival, and the prognostic factor of age may be a surrogate for the gene expression subgroup. As such, the prognostic significance of age may be nullified if controlled for the gene expression subgroup of glioblastoma.
Age, Performance Score, and Extent of Resection
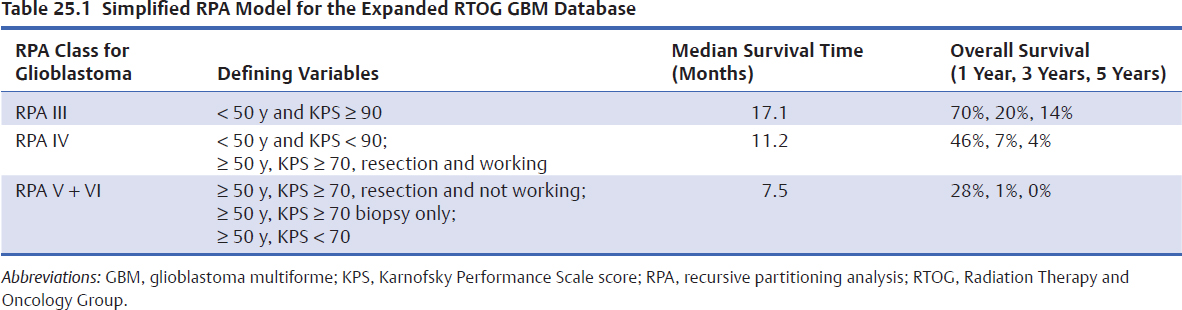
Since its initial development in the early 1990s, the Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis (RPA) classification system has proved useful and has been validated in multiple clinical trials.28–31 It has served as a historical control to compare findings from phase 1/2 clinical trials before phase 3 studies are begun. It also identified relatively homogeneous patient subgroups that may benefit most from a particular experimental approach, thereby sparing other patients from unnecessary treatment. The findings of a recent simplification of the RPA classes for glioblastoma are shown in (Table 25.1).28 The median survival for AO is 3.9 years, with 5- and 10-year survival being 41% and 20%, respectively. For AA, RPA analysis found median survivals of 13, 9, 5.5, and 2.25 years, respectively, from the lowest to the highest RPA risk groups.
Histology
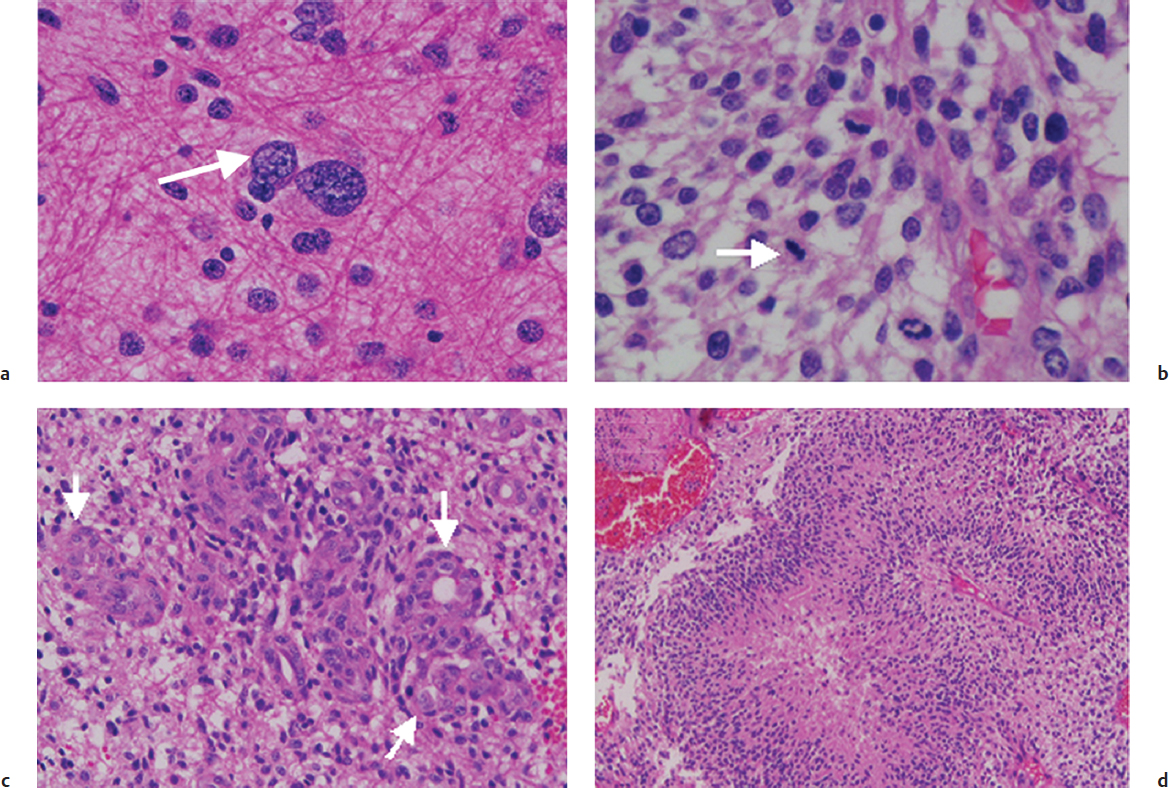
Histologically, AA and GBM both contain pleomorphic nuclei, hypercellularity with mitotic figures, and endothelial prominence (Fig. 25.1). Macroscopically, glioblastomas typically show areas of necrosis and hemorrhage. Differentiating them from AA, glioblastomas typically display what is termed “glomeruloid” vascular endothelial proliferation as well as pseudopalisading necrosis. The designation multiforme, no longer used in the most recent WHO system, was reflective of the extreme pleomorphism of tumor cells, ranging from small and tightly packed to enormous and bizarrely shaped.6
Special Consideration
• Proliferation index (i.e., Ki-67/MIB-1), immunohistochemical staining, and molecular genetic tests are increasingly being used as adjuvant studies for tumor classification and prognostication of malignant gliomas.
Oligodendrogliomas have the characteristic microscopic appearance of round nuclei with sharply defined membranes and inconspicuous chromatin. Formalin fixation yields perinuclear clear halos with the characteristic “fried egg” appearance as well as an array of fine, hexagonal capillaries, which are commonly described as a “chicken wire” pattern. Grade III or anaplastic oligodendrogliomas are distinguished from grade II oligodendrogliomas by the presence of areas of hypercellularity and increased proliferative activity. Oligoastrocytomas display both astrocytic and oligodendroglial components (Fig. 25.2).
Molecular Markers
Although cellular morphology has formed the foundation of brain tumor classification since the days of Cushing and Bailey in the 1920s, and remains the standard for diagnosis in the current WHO scheme, considerable variability in biological behavior and clinical course remains even within individual histological grades. This inherently denotes that a greater degree of complexity on the molecular level than can be appreciated by conventional histopathology underlies the behavior of these tumors. Discoveries related to underlying molecular abnormalities in these tumors have begun to generate new paradigms for understanding how these neoplasms develop and are diagnosed, and how they might be more effectively treated. Currently, the most actively researched individual molecular markers in neuro-oncology are 1p/19q co-deletion in oligodendroglial tumors, alterations in the EGFR and associated signaling pathway genes in glioblastomas, hypermethylation of the MGMT gene promoter in gliomas, and mutations in the IDH1/2 genes in low- and high-grade diffuse gliomas.32
Pearl
• Molecular biomarkers have yielded new insights into the biology of malignant gliomas and their expected clinical course. Patients with tumors harboring methylguanine methyltransferase (MGMT) methylation, IDH mutation, and 1p19q deletion may have a significantly better prognosis than those without these genetic alterations.
1p/19q Chromosomal Co-Deletion
The 1p/19q co-deletion seen in tumors with an oligodendroglial component appears to confer a significant survival advantage and improved response to chemotherapy. This co-deletion results from an unbalanced centromeric translocation of t(1;19)(q10;p10) and can be detected by fluorescence in-situ hybridization (FISH) or polymerase chain reaction (PCR)-based strategies for the detection of loss of heterozygosity. It is frequently seen in oligodendroglial tumors and is present in 80 to 90% oligogdendrogliomas, 60% of anaplastic oligodendrogliomas, and 30 to 50% of oligoastrocytomas.32 This translation appears to have a strong association with classic histological features of round, uniform nuclei with perinuclear halos and a “chicken-wire” vascular pattern. Early studies of patients with this marker found that its presence predicts a better response to chemotherapy with the procarbazine, chloroethylcyclohexylnitrosourea (CCNU; lomustine), and vincristine (PCV) regimen and longer survival in patients with anaplastic oligodendroglioma as well as response to treatment with the alkylating drug temozolomide (TMZ) and radiotherapy.33 However, other retrospective studies have shown benefit regardless of therapy modality, and the marker may therefore be prognostic rather than predictive.34
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree