Fig.
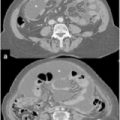
Peritoneal Cancer Index (PCI) classification: Three-dimensional imaging simulation for PCI mapping and scoring. An imaging database can furnish all useful images to place the lesions; dedicated software calculates overall PCI. Images can be used to compare intraoperative and radiological score
Finally, the methods of assessing scores also requires reconsideration; PCI classification refers to the maximum size of tumor implants in a given region but not to the number of implants or total quantity of tumoral mass resulting from the sum of all nodules present in the same region. The same applies when assessing the CC score, which should refer to either the volume of a single residue or the sum of all residues after CRS rather than to the greatest volume of one of several residual neoplastic nodules.
24.2.2 Diagnosis and Staging
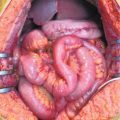
Over the past 20 years, as peritonectomy (PRT) plus HIPEC has progressively become widely used, diagnostic imaging has also shown great technological advancement. Dramatic improvement in the quality of imaging and the possibility of analyzing complex anatomical areas in detail—often significantly altered by size, shape, and conformation of the peritoneal carcinomatosis (PC)—have substantially contributed to diagnosis and staging. Tangible proof of this is the high rate of optimal CRS achieved in all forms of PC treated with peritonectomy procedures. Although the merit of these results should be ascribed to surgeon ability, it is indisputable that an 80–90 % success rate for optimal cytoreduction is also partially attributable to the degree of fidelity and specificity of the technology imaging used in diagnosis.
CT, MRI, and positron emission tomography-CT (PET-CT) are now basic diagnostic procedures, but the contribution of endorectal and vaginal ultrasonography (US), particularly in cases of pelvic organ infiltration, together with clinical evaluation via direct vaginal and rectal examination, should not be disregarded.
Laparoscopy (LPS) occupies a relevant role in the diagnostic phase by allowing direct visualization and biopsy of tumor lesions, which is essential when histological or cytological assessment is not otherwise feasible. The utility of LPS in staging intraperitoneal carcinomatosis, however, is more controversial. This procedure is invasive and can potentially be associated with specific risks; therefore, its use must be correlated against the cost—benefit of the possible result.
PSM staging together with histological analysis is essential in planning a general treatment strategy, as regardless of the classification applied, a high PCI score is an indicator of increased risk of complications and lower survival rates. The most accurate staging is fundamental to surgeons or centers that consider high PCI or Fagotti scores as negative indicators for PRT, whereas it is less important for certain PSM, such as low-grade pseudomyxoma peritonei (PMP) or mesotheliomas, for which the assessment of surgical resectability rather than peritoneal disease extent is more useful.
Those surgeons or centers for which a high level of PCI is not an absolute contraindication for peritonectomy must complete a thorough diagnostic investigation to verify the involvement of sensitive anatomical structures that could render surgical excision inadvisable. The complexity of peritoneal disease and limitations of radiological technologies discussed in the previous chapters may affect the accuracy of diagnosis; however, the indiscriminate use of LPS is questionable and is a reflection on the attitude of surgeons or centers dealing with PSM.
As evident from relevant publications, the mean PCI may differ significantly between studies, but the majority of studies includes significant percentages of cases successfully treated for carcinomatosis staged over the established limits for certain specific PSMs. When staging the extent of PSM, the utility of laparoscopy must be assessed against more traditional morphological investigation procedures and its possible role in identifying the involvement of adjacent anatomical structures constituting negative indicators for excision of the disease, regardless of staging limits. Aside from its valuable diagnostic role under certain circumstances, overall assessment of LPS must also consider the complication and procedure impracticality risks, including risks associated with neoplastic contamination by trocar access sites. The use of LPS, considered by some practitioners as a routine and essential procedure, deserves further evaluation, and prospective studies should be planned to define further its role and potential risks.
24.2.3 Eligibility Criteria
Eligibility criteria regarding the patient’s clinical status before admission to the procedure are rather homogeneous and are extensively discussed in Chap. 13. Over the years, patient age threshold for treating PSM has continuously varied, and at present there is no specific limit; even patients in their 80s and in optimal physical and psychological condition, with easily resectable disease and a good prognosis, are routinely scheduled for the procedure.
The most avidly discussed eligibility criterion is preoperative PC staging, which is particularly relevant in centers in which high peritoneal spread associated with elevated levels of PCI are considered contraindications for CRS. These centers adopt strict selection criteria and more complex diagnostic procedures, which may also involve routine LPS. In centers in which a high extent of PC is not an absolute negative indicator for procedure execution, rather than the presence of unequivocal signs of infiltration of unresectable anatomical structures, staging has a significantly more important role in determining whether neoadjuvant chemotherapy (NACT) should be part of the general treatment strategy.
Progress in molecular biology, genetics, and pathology, as previously described for mesothelioma and OC, as well as chemosensitivity testing applied in the pre-HIPEC phase, will permit more articulated classification of some PSM forms and identification of subtypes, with differing prognoses and variable responses to specific forms of treatment. All these factors will condition eligibility criteria and will guide drug choice for HIPEC and appropriate adoption of NACT. Results from a number of prospective trials in progress at the time of this writing, and improved adherence to multicenter protocols planned on the basis of past experience, will help refine and standardize eligibility criteria, as well as data comparison from case studies.
24.2.4 Peritonectomy
Peritonectomy is a fundamental and irreplaceable procedure in the treatment of PSM. In all forms of PSM and in all settings in which PRT plus HIPEC can be adopted, complete cytoreduction (CC) is the most significant prognostic factor. Maximum cytoreduction improves HIPEC and early postoperative intraperitoneal chemotherapy (EPIC) efficacy and is the main factor responsible for the results widely reported in the previous chapters for survival rates.
No change in treatment orientation is predictable in the near future; maximal cytoreduction is the main treatment goal in all PSM forms and settings. Maximal cytoreduction will always be the preserve of dedicated PSM surgeons and experts in this field with specific training in surgical oncological procedures and knowledge of the rules of peritoneal cytoreduction. More thought, however, should be given to treatment of patients in which PC can be completely or partially reduced by NACT. This treatment option is most applicable to PSM from gastric cancer (GC) and OC, which are PSM forms with the highest application rates of NACT.
The effects of chemical cytoreduction are evident at post-NACT peritonectomy or at second-look surgery, and their evaluation in combination with pre- NACT PC imaging forms the basis for optimizing the choice of CRS. In ovarian carcinomatosis, primary tumor exeresis conducted according to basic radical criteria with total omentectomy, appendectomy, and lymphadenectomy should also include adequate parietal peritonectomy within the lower half of the abdominal cavity. In these cases, peritonectomy must envisage removing the parietal peritoneum from the transverse umbilical line to the pelvis and include pouch stripping. Any other suspicious peritoneal area bearing signs of chemoreduction, such as fibrosis, thickening, and red spots, should be treated with ball-tip electrosurgery or argon in order to alter peritoneal membrane integrity and improve HIPEC efficacy, as previously described.
In gastric carcinomatosis responsive to intraperitoneally and/or systemically delivered NACT, basic radical primary treatment should be associated with appendectomy, bilateral oophorectomy, and extensive supramesocolic parietal peritonectomy, including stripping the peritoneum of the omental bursa, hepatic pedicle, and diaphragms.
Advances in instrumentation technology used in peritonectomy procedures will increase the efficacy and safety in performing carcinomatosis cleanup of complex anatomical areas, expanding limits of resectability and chances of optimal cytoreduction. Training surgeons in these techniques can only be done in highly specialized centers with high-volumes of activity, which permit the acquisition of specific experience over a reasonably short time period.
A laparoscopic approach to this procedure is and will remain a topic of discussion: its role can be considered only for very limited PC.
However, a general lack of information derived from manual assessment in limited carcinomatosis and the difficulty of full abdominal access for comprehensive assessment of diffuse carcinomatosis render the effectiveness of the laparoscopic approach questionable.
A further criticism concerning the laparoscopic approach is neoplastic involvement of trocar access sites, for which conflicting data exist. In this scenario, some conclusions on the subject appear inconsistent and censurable, such as conclusions that metastases of trocar access sites can be easily excised allowing prognosis, similarly to patients subjected to open peritonectomy [4]. Experienced PSM surgeons know very well the severity of tumor diffusion within the abdominal wall, especially when more than one trocar access site is involved, causing complications in treatment and significantly reduced prognosis. Regardless, the laparoscopic approach will be the subject of future controlled studies, which likely will not be conclusive in resolving disagreements among experts in the field.
24.2.5 Hyperthermic Intraperitoneal Chemotherapy
Most criticisms about PSM treatment focus on HIPEC. Except for disseminated MPM (DMPM), PMP, and localized colorectal cancer (CRC) carcinomatosis, the use of PRT plus HIPEC for other types of PSM is questioned mainly in relation to potential morbidity and therapeutic efficacy.
Scepticism regarding HIPEC is supported mainly by the lack of RCTs, which are complicated to conduct; thus, HIPEC is supported mainly on a strong rationale for its use and on available results derived almost exclusively from phase I and II studies. Although PRT plus HIPEC is considered the treatment of choice for few PSM, its application is progressively increasing for a wider variety of PSM forms.
HIPEC techniques are described in Chap. 9 and in subsequent chapters, as is its role in combination with CRS for treating major forms of PSM. Overall results drawn from past experience in all forms of PSM, including the meagre data from prospective studies, appear to validate the benefits in terms of survival compared with traditional treatments but do nothing to counteract the vehement criticism and scepticism regarding HIPEC. Indeed, the perception among sceptics is that only peritonectomy, undertaken as maximum cytoreduction, is the commonly accepted procedure by which to improve survival rather than traditional treatment based on salvage surgery and systemic chemotherapy or, more rarely, intraperitoneal normothermic chemotherapy. Pending results of randomized prospective studies on the role of HIPEC in treating various types of PSM, HIPEC continues to be widely used, and the various related studies concerning the development of new technologies and new drugs are clear proof of the scientific community’s interest in this method.
HIPEC techniques, described in detail in Chap. 10, analyze the difference between open, closed, or semiclosed methods. To date, all studies agree that the choice of technique does not influence procedure efficacy or indicate any need for controlled studies in this regard. The choice is therefore left to the operators, who must also take into account environmental assessments and logistic factors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree