1
Prevalence
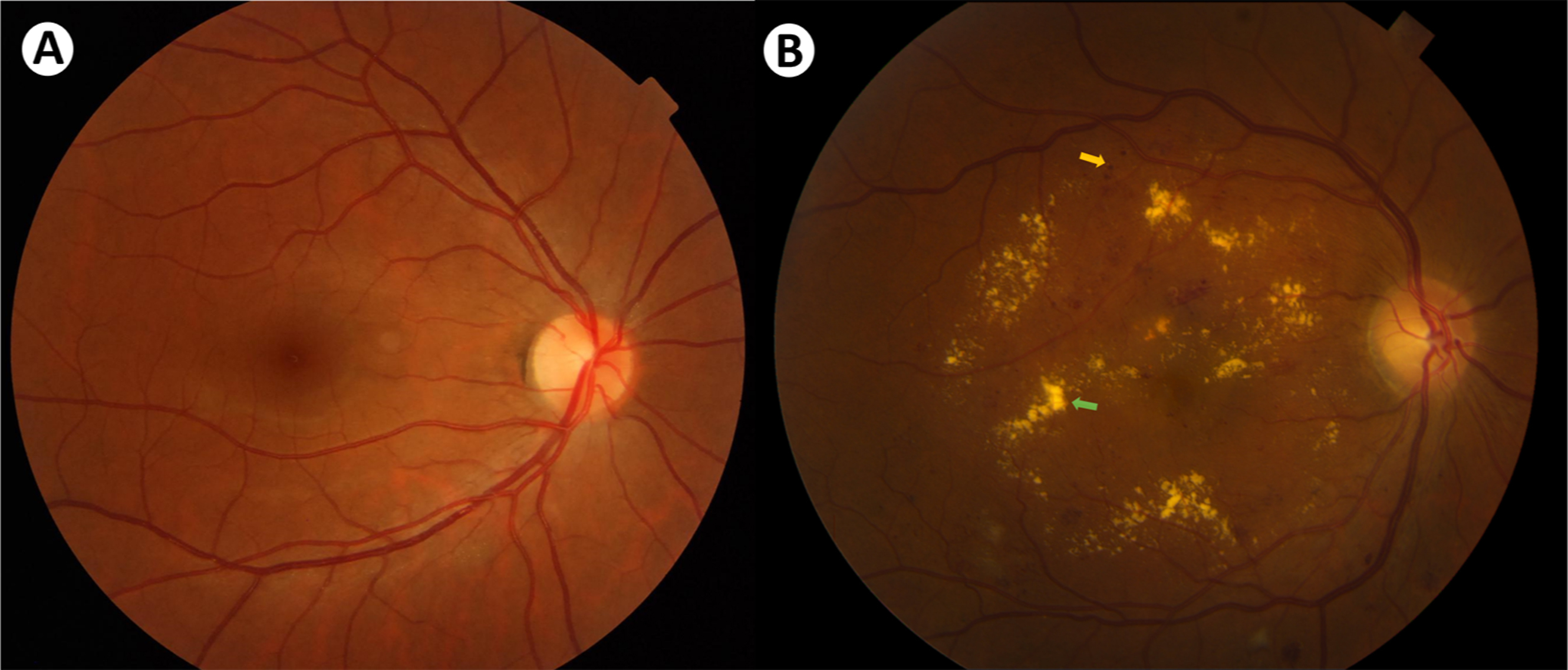
Diabetes mellitus (DM) is a chronic metabolic disorder that has reached epidemic proportions with a global prevalence of 9.3% and an expected rise to 25% by 2030 [ , ]. Chronic hyperglycemia, the hallmark of DM, damages the vasculature leading to both macrovascular and microvascular complications. The most common microvascular complication of DM is diabetic retinopathy (DR) with an annual global incidence of up to 12.7% [ ] and global prevalence of 34.6% [ ]. Diabetic macular edema (DME) is a highly prevalent complication of DR with a worldwide prevalence of 12.8% [ ]. DME is characterized by fluid accumulation in the macula, which can occur at any stage of DR although it is more common in more advanced stages Fig. 7.1 [ ]. Since DME is a major cause of preventable vision loss, a clear understanding of its risk factors and pathogenesis is necessary to improve current management and provide novel alternative treatments [ ].
2
Risk factors
The major risk factors associated with DME include duration of DM, elevated blood sugar levels, elevated blood pressures, and dyslipidemia [ , ]. The increased risk of DME associated with the duration of DM has been borne out by landmark clinical trials [ , ].
In parallel, hyperglycemia is another major risk factor [ ]. The Diabetes Control and Complications Trial compared the effect of intensive blood glucose control on the risk of DR compared with conventional treatment. The authors found that intensive blood glucose control can decrease the risk of DME by 46% [ ]. Despite the fact that HbA1c levels from the two groups converged shortly after the study closeout, patients from the initial intensive glucose control group continued to enjoy reduced risk of DME: 58% at 4 years [ ], 44% at 10 years [ ], and 23% at 18 years, at the time when the long-term benefit started to fade [ ]. Similarly, in the Wisconsin Epidemiologic Study of Diabetic Retinopathy, lowering HbA1c levels by 1% were associated with a 25% reduction in the risk of DME at 10 years [ ] and 22% reduction at 25 years [ ]. Moreover, compared with the duration of DM, hyperglycemia seems to have a more pronounced effect on the risk of DME in patients who already have diabetic retinopathy [ ].
Another major modifiable risk factor is hypertension [ ]. Tight blood pressure control (<150/85 mm Hg) can reduce the risk of DME compared with less tight control (<180/105 mm Hg) [ ]. However, this benefit is not sustained after stopping blood pressure control, highlighting the critical role of patient compliance [ ]. A 2015 Cochrane systematic review also showed that tight blood pressure control reduced the risk of DR development but not the progression of DME. Considering the modest support for blood pressure control to prevent DR and the associated risk of hypotension, the authors concluded that available evidence does not justify blood pressure reduction only for the purpose of DR prevention [ ].
In addition to hyperglycemia and hypertension, dyslipidemia also contributes to DME, but the evidence remains inconsistent. A cross-sectional study of 500 diabetic patients found that serum lipids are associated with clinically significant macular edema (CSME) but not DR [ ]. A recent systematic review, however, found that the strong relationship between lipid levels and DME was shown in cohort and case-control studies but was not confirmed by prospective interventional randomized clinical trials (RCTs) using lipid-lowering agents [ ]. The FIELD study was an RCT that evaluated the effect of fenofibrate on the need for laser treatment for DR, including DME. The study showed that fenofibrates reduced the need for laser therapy although this benefit might not be related to lipid serum levels [ ]. Therefore, further investigation is warranted to evaluate the role of lipid levels and lipid lower agents in DME. In fact, the DRCR.net is currently recruiting for protocol-AF study (NCT04661358), a phase III RCT that evaluates the role of Fenofibrate in preventing progression of DR. Protocol-AF study will compare the effect of Fenofibrate (160 mg once daily) to placebo on the progression of DR through 4 years of follow up. The study addresses patients with mild to moderately severe nonproliferative DR who do not have center involved DME (CI-DME) at baseline [ ].
Sleep apnea has also been recognized as a risk factor that may increase the risk of DME and treatment resistance in patients with type 2 DM [ ].
Ethnic background might also influence the risk of DME. A large cross-sectional study in the USA found that among non-Hispanic patients with diabetes, black subjects were at higher risk of DME as compared with whites [ ]. Moreover, the Singapore Epidemiology of Eye Diseases study showed that Indians were at higher risk of any DR, including DME [ ]. The difference among ethnic groups suggests potential genetic or environmental influences in the development of DME. Indeed, several studies have found a significant association between multiple single nucleotide polymorphisms of vascular endothelial growth factor (VEGF) and DME [ ]. However, the role of genetics in DME is not well established, and further studies are required to gain a better understanding [ ].
Despite our improved understanding of risk factors, they remain poor predictors of DME outcome rendering medical intervention insufficient on its own. For instance, 1% reduction in HbA1c and a 10 mm Hg decrease in systolic blood pressure reduced the risk of DME only by 17% and 15%, respectively [ ]. Also, in the Diabetes Control and Complications Trial evaluating the benefits of intensive glucose control on the risk of DR, other time-varying covariates accounted for less than 37% of the risk of DR progression [ ]. However, a comprehensive approach is necessary because medical treatment to control these risk factors remains critical to delay the onset of DME and the need for local ocular interventions.
3
Normal retinal fluid homeostasis
Normal fluid homeostasis in the retina is necessary to maintain normal cellular metabolism, tissue architecture, and optical transparency and is achieved by a balance between fluid entry and removal. The inner blood–retinal barrier (BRB) prevents leakage from retinal vasculature and consists of several components [ ]. Endothelial cells lining the inner vascular wall are connected by tight junctions that restrict the passage of molecules across the paracellular route [ ]. Besides endothelial cells and their junctions, pericytes represent a second line of defense of the BRB. Pericytes are mural cells that tightly wrap around the retinal vasculature with an overall higher coverage of the retinal than the brain vasculature [ , ]. In addition to pericytes, glial cells are pivotal in the regulation and maintenance of the BRB. Glial cells constitute two groups: macroglia (includes Muller cells and astrocytes) and microglia. Macroglia wrap around retinal capillaries and play a major role in the development and maintenance of BRB [ , ], whereas microglia represent a localized immunocompetent cell population that also play a major role in maintaining an effective BRB [ ].
In addition to the inner BRB, the intercellular junction complex of the retinal pigment epithelium (RPE) and the outer limiting membrane (OLM) form another important barrier to fluid entry into the retina: the outer BRB [ , ]. Besides its pivotal role in outer BRB, the RPE also contributes to fluid clearance from the retina into the choroid. Active transport is the major absorption mechanism by the RPE (70%), where water is osmotically driven into the choroid by a chloride gradient and through aquaporin channels [ ]. Another major component of fluid clearance is the Muller cells. These cells express membrane aquaporins that permit fluid passage following osmotic gradients [ , ]. Similar to the RPE, fluid transport is largely driven by chloride transport, which is mediated by various channels [ , ].
4
Pathogenesis of DME
Disruption of any component of the tightly regulated BRB can contribute to abnormal accumulation of fluid in the retina leading to macular edema Fig. 7.2 . Chronic hyperglycemia activates numerous pathways that can potentially disrupt retinal fluid homeostasis in diabetic macular edema [ ].
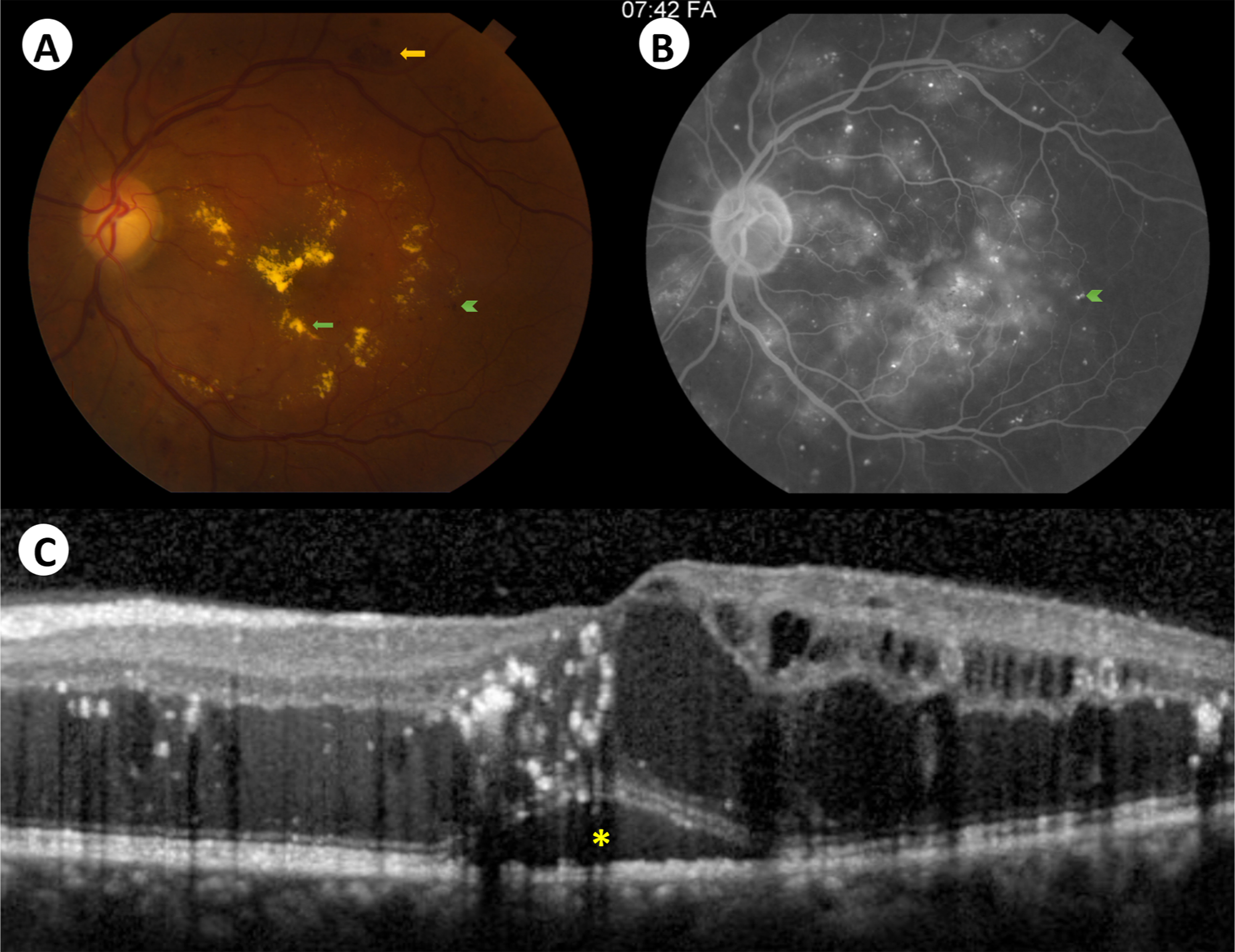
The polyol/sorbitol pathway is upregulated in chronic hyperglycemia, leading to oxidative and osmotic stress, and accumulation of advanced glycation end products (AGEs) [ ]. The protein kinase C (PKC) pathway is also upregulated, and along with AGEs, results in pericyte apoptosis [ ], barrier permeability [ ] and inflammation [ ], and eventually, barrier dysfunction [ ].
VEGF also plays a major role in the development of DME [ ]. Retinal hypoxia is well documented in large animal models of DR [ ]. In hypoxic retinal tissue, hypoxia inducible factor is upregulated leading to the overexpression of VEGF [ , ]. Elevated VEGF concentration in the aqueous and vitreous humor of diabetic eyes correlates with DME severity [ , ]. This is explained by the effect of VEGF on the BRB components. VEGF increases paracellular endothelial cell permeability by reducing the tight junctions and altering their structural components via PKC dependent pathways [ ]. Also, VEGF increases transcellular transport by upregulating endothelial transcytosis [ ]. VEGF drives several pathological pathways and is also upregulated by other mechanisms, such as hyperglycemia, PKC, and inflammatory cytokines [ , , , ], demonstrating the complexity of DME pathophysiology.
Endothelial cell loss can also be exacerbated by VEGF-induced leukocyte adhesion to retinal vasculature [ , ]. VEGF can also reduce tight junction expression [ ] and induce its structural modification by phosphorylation [ ]. Moreover, several other mechanisms contribute to the loss of pericytes, a hallmark of DME. These include PKC pathway [ ], angiopoietin pathway [ ], and pro-inflammatory pathways [ ].
Besides barrier disruption, dysfunction in the fluid removal mechanisms from the retina is another major component of DME pathogenesis. Muller cells fluid clearance mechanism is impaired in DM [ ]. Immunohistochemical studies of retinal tissues in diabetic rats showed altered expression and distribution of aquaporin channels in Muller cells [ ]. Also, the subcellular distribution of K+ channels is altered leading to reduced K+ conductance, intracellular K+ overload, and osmotic cell swelling, further compromising its function and drawing more fluid from the blood and vitreous [ , ]. Moreover, RPE capacity to absorb fluid is also impaired in DM, contributing to fluid accumulation in the retina. Studies in diabetic animal models have shown that hyperglycemia reduces the RPE Na+/K+ -ATPase activity [ , ] and alters the expression of various aquaporin isoforms in the RPE [ ]. The dysfunction in ion and water transport across the RPE contributes to the accumulation of retinal fluid in DME [ ].
5
Treatment
5.1
Anti-VEGF therapy
Given the pivotal role of VEGF in the pathogenesis of BRB breakdown, and its high levels in the vitreous humor of eyes with DR [ ], anti-VEGF agents have played a central role in the management of DME. Indeed, anti-VEGF is currently the first-line treatment for CI-DME, supported by randomized controlled studies showing superior efficacy compared with argon laser photocoagulation [ ]. CI-DME is best detected on optical coherence tomography and was defined by the DRCR.net as central subfield thickness more than two SDs above the average thickness of a normative cohort of patients with DM but no or minimal DR Fig. 7.3 [ ].
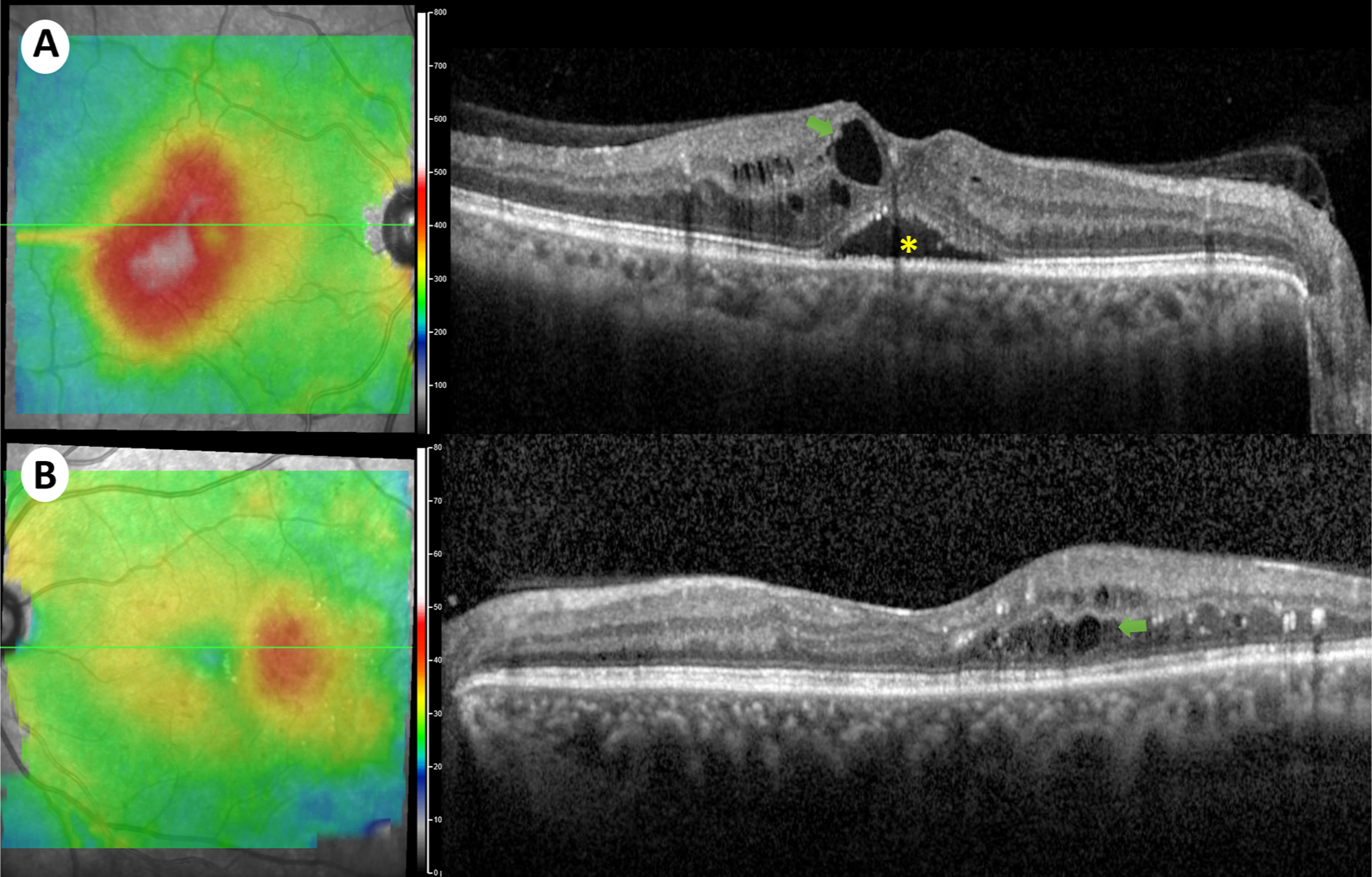
Protocol T study was a comparative efficacy RCT conducted by the DRCR.net to compare the efficacy of the three anti-VEGF agents in the treatment of CI-DME. This study compared the outcomes of aflibercept (Eylea, Regeneron Pharmaceuticals), ranibizumab (Lucentis, Genentech), and bevacizumab (Avastin, Genentech), with the primary outcome of mean change in visual acuity at 1 year [ ].
Protocol T treatment algorithm consisted of initial 6-monthly loading dose (monthly injections for the first 6 months) unless “complete response” or “no improvement/worsening” occurred. Starting at 24 weeks, eyes with “no improvement/worsening” after two consecutive injections, regardless of their visual acuity and central subfield thickness, had their injections withheld; treatment was resumed if visual acuity or central subfield thickness worsened. Complete response was defined as visual acuity of 20/20 or better with central subfield thickness below the eligibility threshold (250 μm). Improvement/worsening was defined as visual acuity letter score increase/decrease of five or more or central subfield thickness increase/decrease of 10% or more.
Protocol T study showed that all three anti-VEGF agents were effective in improving vision, with interesting differences that were largely driven by baseline visual acuity. When the baseline visual acuity loss was mild (20/32-20/40), all three drugs had a similar effect at 1 and 2 years follow up. However, when baseline visual acuity loss was more severe (20/50 or worse), aflibercept was more effective at improving vision at 1 year; at 2 years, ranibizumab did “catch-up” and demonstrated similar efficacy to aflibercept, and both were superior to bevacizumab [ ].
The treating physician’s decision on the choice of treatment agent, and regimen should thus be multifactorial. To choose the appropriate agent, physicians must consider several factors, including baseline visual acuity, efficacy, cost, and safety.
Based on the findings from the DRCR.net protocol T study, when treating patients with mild baseline vision loss, any of these agents would be a reasonable initial choice. On the other hand, in patients with low initial visual acuity, it is reasonable to consider aflibercept as a first-line therapy. In the 3-year follow-up of VISTA and VIVID studies, eyes in the laser group received delayed aflibercept injection and did not achieve similar visual outcomes to eyes initially treated with aflibercept suggesting a possible irreversible vision loss because of delayed initiation of aflibercept treatment in the laser control group [ ]. The superior efficacy of aflibercept is aligned with its higher binding affinity to VEGF as shown in pharmacodynamic studies [ ].
To compare the cost-effectiveness of each anti-VEGF agent, Ross et al. conducted a post-hoc analysis of the protocol T trial and compared their incremental cost-effectiveness ratio defined as the ratio of incremental cost (in 2015 USD) to its incremental benefit (in quality-adjusted life-years) [ ]. They concluded that aflibercept and ranibizumab are not cost effective compared to bevacizumab at this time.
Anti-VEGF treatment is generally considered safe, and the overall safety profile of all three agents is similar, as shown by a recent Cochrane systematic review (moderate-to high-certainty evidence) [ ]. However, some studies showed a slightly increased risk of systemic adverse events, but these findings should be considered with caution. For instance, a meta-analysis of DME patients receiving monthly anti-VEGF for 2 years showed an increased risk of death and cerebrovascular accidents with such an aggressive treatment regimen [ ]. Furthermore, in the DRCR.net protocol T study, ranibizumab demonstrated a higher cardiovascular risk over 2 years, measured by Anti-Platelet Trialists’ Collaboration (APTC) events, compared with aflibercept and bevacizumab [ ]. However, this finding was inconsistent with other studies [ ]. Moreover, some concern has been raised about the repackaging of bevacizumab and its safety. Although compounded bevacizumab has been associated with case reports of endophthalmitis, high-quality studies did not show an increased risk with the current distribution protocol in the USA [ , ].
Besides choosing the treatment drug, clinicians must decide on the treatment protocol. These potential regimens include aggressive initial treatment followed by monitoring, treat and extend, pro re nata (PRN), and deferring initial treatment. The DRCR.net protocol employs four monthly injections during the first 4 months, followed by injections if “success” was not achieved during the subsequent 2 months. After the sixth month, eyes that “improved” since the last injection are treated. During the second year, treatment interval can be doubled if injection is withheld at three consecutive visits [ ]. The DRCR.net protocol has been shown to achieve vision gains with progressively fewer annual injections compared with fixed more aggressive treatment regimens [ ].
Another treatment approach is the treat and extend protocol. Aflibercept treat and extend protocol has been evaluated in the VIOLET and EVADE studies. In the VIOLET study, patients with CI-DME that have been treated with aflibercept for at least 1 year were randomly assigned into three subgroups with different treatment intervals: fixed 2-month, PRN, and treat and extend. All three groups were able to maintain gains in visual acuity. In the EVADE study, the treat and extend regimen was compared to a fixed regimen (5 initial monthly injections followed by 2-monthly injections). The treat and extend group experienced better visual gains at 1 year, in association with a higher number of injections received [ ]. Ranibizumab has been evaluated in the RETAIN study, where patients with DME were randomized into three treatment regimen groups: treat and extend (with or without laser) and PRN. Treat and extend regimens were noninferior to PRN based on visual gains with less clinic visits [ ]. The TREX-DME trial compared treat and extend algorithm of ranibizumab with monthly dosing regimen and demonstrated similar visual and anatomic gains over 2 years with the advantage of reduced number of injections in the treat and extend group [ ]. Aflibercept PRN protocol has been evaluated in the DAVINCI trial that compared monthly, two-monthly, and PRN regimens of aflibercept with laser. The study showed that all regimens demonstrated similar visual gains over 1 year and were superior to laser [ ].
The majority of patients with DME (80%) present with a good best corrected visual acuity (BCVA) (20/40 or better) [ ]. Therefore, deferring initial treatment while monitoring these patients closely is another attractive approach. To evaluate the initial treatment deferral, the DRCR.net conducted an RCT to compare aflibercept, laser, or observation as initial treatment approach in patients with CI-DME and good visual acuity (20/25 or better). The authors concluded that there was no significant difference in vision loss at 2 years across the three groups, supporting initial treatment deferral with close follow-up in eyes with good baseline visual acuity until visual acuity decreases [ ].
Anti-VEGF ocular injections are generally considered to be safe, but ocular and systemic side effects remain a risk, especially with treatment regimens that require multiple injections over extended periods. Among the ocular complications, the most feared—albeit rare—is infectious endophthalmitis [ ]. The DRCR.net found that all patients who received anti-VEGF injections without prior administration of povidone-iodine developed infectious endophthalmitis during the course of treatment [ ]. Furthermore, topical prophylactic antibiotics were found to be unnecessary, or even harmful, for preventing infectious endophthalmitis [ ]. Therefore, conjunctival antisepsis with povidone-iodine and elimination of topical antibiotic during anti-VEGF injection is recommended, as supported by high-quality studies [ , ]. Other ocular adverse effects include intraocular inflammation, rhegmatogenous retinal detachment, elevated intraocular pressure, and subconjunctival hemorrhage [ , ]. A recent systematic review showed that although IOP rises just after anti-VEGF injection, it was lower than baseline 1 day later and did not show any significant difference in the long term [ ].
Besides ocular complications, systemic adverse events remain a concern especially that systemic VEGF levels decrease after intravitreal anti-VEGF injections suggesting its diffusion into the systemic circulation [ , ]. Systematic reviews reported a possible increased risk of death, cerebrovascular accidents, venous thromboembolism, and nonocular hemorrhagic events in older patients [ , ]. However, a recent Cochrane systematic review and meta-analysis showed that anti-VEGF agents have good safety profile and do not differ regarding systemic serious adverse events (moderate- or high-certainty evidence) [ ].
Despite the variety of treatment options available and the high efficacy of anti-VEGF for DME, many eyes remain unresponsive or partially responsive to treatment Fig. 7.4 . In the DRCR.net landmark study, only around one third of the eyes treated with ranibizumab plus prompt/deferred laser achieved complete visual response (20/25 or better) at 1- and 2-year follow-up [ ]. Furthermore, the protocol T 5-year follow-up study showed that more than half the patients (53%) did not recover or maintain complete visual acuity (20/25 or better) at 5 years [ ]. In fact, mean visual acuity decreased by 4.7 letters between 2 and 5 years, after the study was completed [ ]. On the other hand, the protocol-I 5-year follow up study, with continued structured retreatment protocol, showed that visual improvements at 1 year were maintained through the 5 years with less injections required [ ]. Therefore, close long-term monitoring is clearly necessary to maintain the initial visual gains.
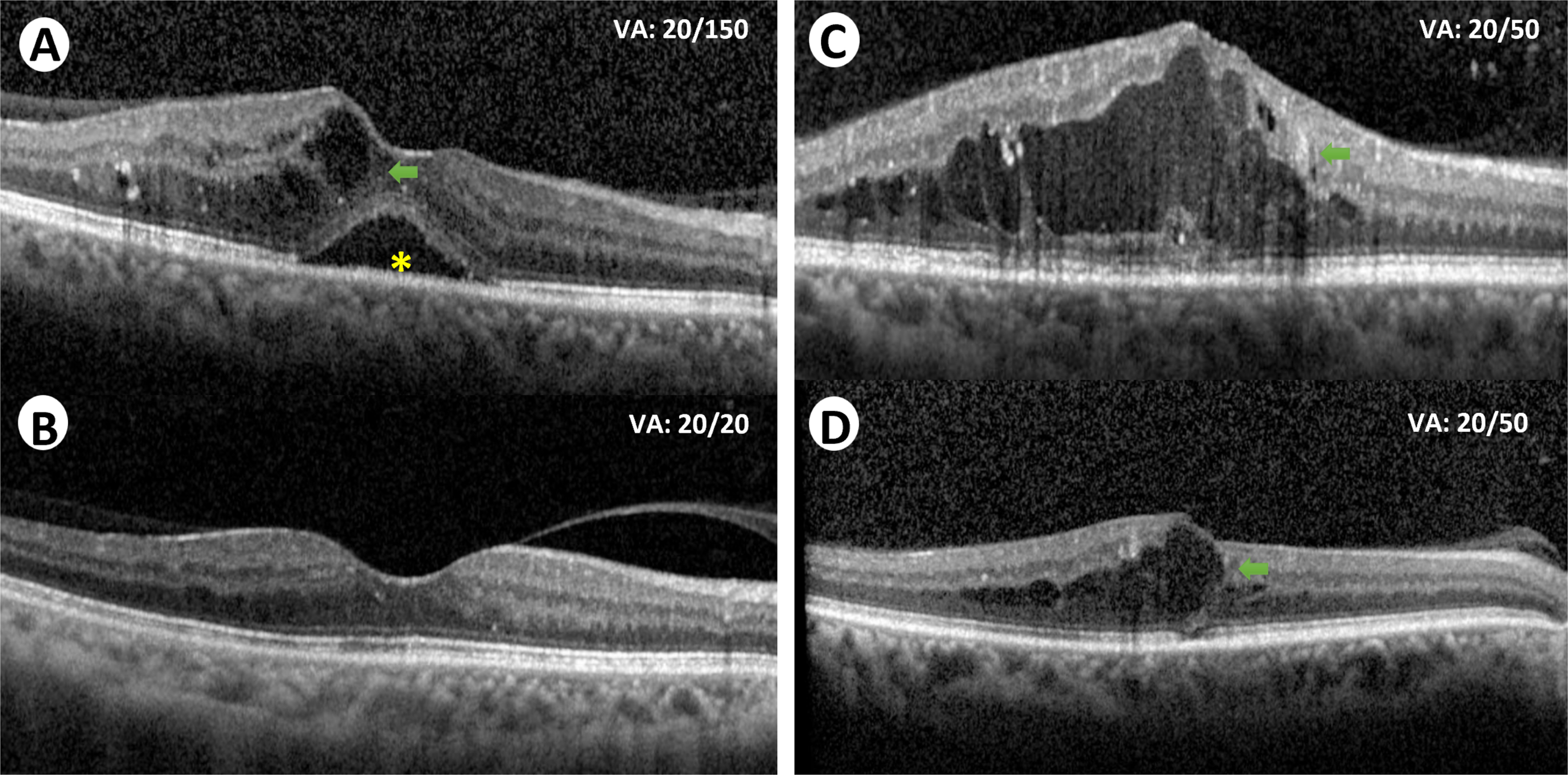
To treat patients with persistent CI-DME, the DRCR.net conducted protocol U study to explore the benefits of adding dexamethasone to continued ranibizumab treatment in these patients. The authors concluded that no vision improvement was achieved despite a decrease in the central subfield thickness over 6 months [ ].
An alternative approach to achieve better visual outcomes in persistent DME is to switch anti-VEGF agents; however, the definition of persistent/unresponsive DME is unclear in the literature and varies across switching studies, rendering the evaluation of this approach challenging. In the post-hoc analysis of protocol T study, the DRCR.net suggested that early switching is not recommended given the continuous improvement in visual and anatomic outcomes after 3 months of treatment initiation [ ]. However, because persistent DME was more likely with bevacizumab than aflibercept and ranibizumab, switching from bevacizumab might be plausible later during treatment [ ]. A retrospective study of eyes with persistent DME previously treated with bevacizumab showed improved outcomes upon switching to other agents [ ]. Furthermore, recent meta-analyses showed that switching to aflibercept in eyes with bevacizumab or ranibizumab treatment resistant DME showed significant improvement in visual and anatomical outcomes [ , ]. Overall, given the heterogeneous treatment responses among patients, and the visual acuity decline during extended follow up [ ], current research is seeking to find better approaches to treat unresponsive eyes and to maintain long-term visual outcomes in responsive eyes.
5.2
Laser photocoagulation
The exact mechanism of action of laser in DME remains unclear, but it might involve the closure of leaky capillaries, and the activation of the RPE to restore the BRB, as reviewed by Bhagat et al. [ ]. Before the advent of anti-VEGF therapy, laser treatment had been the gold standard treatment for DME. In the Early Treatment Diabetic Retinopathy Study (ETDRS), treating eyes with CSME (retinal thickening involving or threatening the center of the macula) with focal laser decreased the risk of moderate visual loss (loss of >15 letter score) by half [ ]. Furthermore, focal/grid laser is more beneficial for CSME than intravitreal triamcinolone. The protocol B study conducted by the DRCR.net showed that focal/grid laser for DME involving the fovea was more effective and had less adverse effects over 2 years, compared with intravitreal triamcinolone [ ]. However, laser is inferior to anti-VEGF in the treatment of CI-DME. In a subsequent study by the same group, ranibizumab demonstrated better visual outcomes over 2 years when compared with focal/grid laser monotherapy [ ]. Anti-VEGF therapy generally targets CI-DME. Therefore, for eyes with noncenter involved DME, focal laser can be used to directly target leaking microaneurysms away from the fovea [ ]. The modified ETDRS laser protocol is currently the recommended technique, and it entails using smaller and less intense laser to target all leaking microaneurysms in areas of retinal thickening while avoiding the center of the macular within 500 μm [ ].
The DAVE study was a 3-year phase I/II RCT that evaluated the benefits of treating peripheral retinal nonperfusion identified by widefield fluorescein angiography (200°) with targeted retinal photocoagulation in patients with DME. Interestingly, this study did not find evidence to support the treatment of peripheral nonperfusion in these patients [ ].
Focal/grid laser photocoagulation therapy is safe especially when used carefully and appropriately. However, cases of reduced contrast sensitivity, paracentral scotoma, laser scar expansion, subretinal fibrosis, and choroidal neovascularization have been reported [ ]. As a potentially less damaging alternative, subthreshold micropulse photocoagulation is currently being evaluated in RCTs [ , , ].
5.3
Novel therapies
Anti-VEGF treatment provides significant visual gains for patients with CI-DME, but multiple injections are required and sustained visual outcomes are not guaranteed in the long term [ ]. Therefore, alternative agents with extended effect as well as drugs that targets VEGF-independent pathways are needed. Indeed, several novel therapies are currently in the pipeline.
To reduce the number of injections and the burden on the patients and the healthcare system, high-dose aflibercept dosed at longer intervals is a plausible alternative to the available treatment approaches. The PHOTON study (NCT04429503) is a phase II/III RCT that is currently active, and its primary objective is to determine if high-dose aflibercept (8 mg) dosed at 12- or 16-week intervals is noninferior to the 8 weeks interval dosing regimen of the conventional approved dose (2 mg), with respect to BCVA [ ].
Brolucizumab (Novartis) is another small size anti-VEGF agent that has been approved for wet age-related macular degeneration, and several studies are evaluating its efficacy for DME [ ]. However, the risk of intraocular inflammation, retinal vasculitis, and vascular occlusion reported in the wet AMD phase III RCTs HAWK and HARRIER [ ], as well as in postmarketing reports [ , ], has raised concerns regarding its safety profile. Physicians should weigh potential benefits against these risks when considering brolucizumab as a treatment option for DME subjects.
Faricimab (Genentech/Roche) is a bispecific monoclonal antibody that binds both VEGF-A and angiopoietin-2 [ ]. The BOULEVARD Phase II study (NCT02699450) demonstrated higher visual gains at 24 weeks in treatment naïve patients with CI-DME who received faricimab compared with ranibizumab [ ]. Several phase III RCTs are evaluating the long-term efficacy and safety of faricimab in patients with DME .
Port delivery system with ranibizumab allows sustained release of the drug into the vitreous with fewer injections required. PAGODA study (NCT04108156) is a phase III noninferiority study comparing port delivery system with ranibizumab refilled at 6-month intervals on one hand, with monthly ranibizumab injections on the other hand [ ].
Other novel therapies in the pipeline include gene therapy and other cytokines inhibitors. For instance, ADVM-022 is a recombinant, adeno-associated virus gene therapy vector that encodes for aflibercept, providing hope for sustained anti-VEGF release and fewer required injections [ ]. The INFINITY trial (NCT04418427) is a phase II RCT currently evaluating the durability of single intravitreal injection of ADVM-022 compared to aflibercept in patients with DME [ ].
6
Conclusion
DME is a major vision-threatening, but treatable complication of DM, with current therapeutic options that are able to prevent and sometimes reverse vision loss in a majority of eyes. Understanding the risk factors for DME enables clinicians to address these systemic issues in affected patients, as important adjuvants to local ocular treatment. New insights on the pathophysiology of the disease have allowed the development of anti-VEGF agents as an effective treatment for DME. Despite its efficacy, anti-VEGF treatment is associated with several challenges. Novel therapies that deal with these challenges are currently in the pipeline.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree