Represents ˜12% of lymphomas
˜8-9K cases annually in US, resulting in ˜1300 death
Typically affects younger pts (20s-30s) but can also affect older adults
2 major types:
Classical HL
Classical HL characterized pathologically by the Reed-Sternberg Cell, a large, bilobed, or multinucleated cell that classically has an “owl’s eyes” appearance & stains (+) for CD15 & CD30
Subtypes of Classical HL include: Nodular sclerosis (most common), lymphocyte rich, mixed cellularity, & lymphocyte depleted. Tx does not differ among these subtypes
NLPHL is a more indolent disease characterized by the Lymphocytic & Histiocytic cell, a Reed-Sternberg variant that stains negative for CD15 & CD30, but (+) for CD20
Echo if anthracycline to be used, PFTs if bleomycin to be used
Cotswolds modification of the Ann Arbor Staging System (Lister TA, et al. J Clin Oncol 1989;7:1630)
|
The IPS includes 7 RFs for adverse outcome (N Engl J Med 1998;339:1506):
Serum albumin <4 g/dL
Hb <10.5 g/dL
WBC ≥15K
Age ≥45
Male sex
Stage IV
Lymphocyte count <600/mm3 or <8% of WBC
Other RFs include: B-sx, elevated ESR, bulky disease, mixed-cellularity or lymphocyte depleted histology, abdominal or pulmonary hilar involvement & increasing number of nodal sites
Includes Stages I-IIA, nonbulky
Standard combined modality Rx w/2-4 cycles of ABVD & 20-30 Gy ISRT results in PFS ˜85% & OS >90% (J Clin Oncol 2003;21:3601; N Engl J Med 2012;366:399).
ABVD = doxorubicin, bleomycin, vinblastine, & dacarbazine
Additional RT (10 Gy) is given to sites of bulky disease
2 cycles of ABVD & 20 Gy of IFRT equally effective in a select group of early stage pts (“early favorable”) who have no clinical RFs (N Engl J Med 2010;363:640)
Repeat PET post-tx for response assessment
“Adv Stage” includes stages III-IV. Some groups include bulky disease of any stage and/or Stage IIB w/unfavorable factors
ABVD chemotherapy for 6 cycles is standard in North America. Pts w/bulky disease receive ISRT of 30-36 Gy
Escalated BEACOPP improves FFTF & possibly OS in pts w/unfavorable RFs (eg, IPS ≥4), but is more toxic than ABVD (N Engl J Med 2003;348:2386)
Limited data for benefit of consolidative RT (J Clin Oncol 2004;22:62) but typically reserved for bulky disease in current era of improved salvage Rx
Most pts should receive salvage chemotherapy such as ICE or DHAP ± accelerated TLI/STLI w/boost (J Clin Oncol 1993;11:1062) followed by consolidative autologous stem cell transplant if responsive
RFs for adverse outcome post relapse (Blood 2001;97:616):
Remission duration <12 mos
B-sx
Extranodal disease
Role of allogeneic transplant is unclear
Brentuximab vedotin has 75% RR in post-ASCT setting
Multiple combination chemotherapy regimens have reported activity in multiply relapsed pts
Annual breast screening should begin 8-10 y after RT that included some of the breast (no later than age 40) in women who receive it. MRI together w/mammography for women that receive RT between ages 10 & 30 is the standard screening for women at high risk for breast CA
Annual chest imaging recommended for pts at increased risk of lung CA due to tobacco use, receipt of alkylating agents, or chest RT
Cardiovascular RFs should be screened for & modified
Most common indolent lymphoma & 2nd most common lymphoma overall, representing ˜25% of NHLs
More common in Caucasians than other races, incidence ↑ w/age. Equal incidence in men vs. women
Composed primarily of small cleaved lymphocytes that grow in a follicular pattern resembling that of nl 2° lymphoid tissue
Variable numbers of larger centroblasts are typically present, w/↑ number indicating higher grade
Immunophenotypically, FL cells classically express the B-cell Ags CD-19 & CD-20, the follicular center Ag CD-10, & the anti-apoptotic protein BCL-2. They do not express T-cell Ags, including CD-3 or CD-5 (the latter is often aberrantly expressed in CLL/SLL or MCL)
Overexpression of BCL-2, an anti-apoptotic protein, is mediated by the t(14:18) w/c juxtaposes the BCL-2 gene to the Ig heavy chain locus in a large majority of cases. Translocation not sufficient to diagnose FL
Ann Arbor Staging w/Cotswolds modifications, as for HL (J Clin Oncol 1989;7:1630)
Staging primarily consists of cross-sectional imaging w/either a CT scan of chest/abdomen/pelvis ± neck or a PET scan
PET particularly useful if there is concern for transformed disease or to confirm limited distribution in early stage
BM bx needed to complete staging, but can be deferred if observation or standard Rx for adv disease is planned
As w/other lymphomas, stage alone confers limited prognostic info
2 main prognostic models have been reported: The FLIPI (Blood 2004;104:1258) & the FLIPI2 (J Clin Oncol 2009;26:4555)
Generally considered incurable w/standard therapies, & no survival advantage has been demonstrated w/earlier initiation of Rx
Observation alone is recommended for most asymptomatic pts
Observed pts are usu followed w/serial clinical exams & periodic CT (not PET). Optimal timing of scans not determined, may vary according to individual pt characteristics
Median time to initiation of Rx in observed pts is ˜3 y
Rituximab monotherapy increases time to first chemotherapy, but not survival (ASH Annual Meeting 2010;116:Abstract 6)
Enrollment in clinical trial should be encouraged
Commonly accepted indications for Rx include: Any node >7 cm, or 3 nodes >3 cm, sx, cytopenias, fluid collections (ascites, pleural effusions), organ impairment & leukemic phase of disease (J Clin Oncol 1998;16:2332)
Multiple front-line therapeutic options. Choice of Rx is based on pt & disease characteristics, including: Volume & distribution of disease, age, & functional status of the pt, potential need for rapid response, potential concern for transformed disease (see below)
Rituximab is a monoclonal Ab directed at the B-cell Ag CD-20. Efficacy has been shown w/both single-agent & combination Rx
Rituximab monotherapy (4 weekly doses) is used 1° in pts w/low volume disease. ORR ˜70-75%, CR ˜40-45% in untreated pts (J Clin Oncol 2005;23:1103)
Asymptomatic relapsed pts can be observed, as w/untreated pts
Choice of 2nd-line Rx influenced by degree & duration of response to initial Rx. Front-line regimen can be re-used if remission duration was long
Rituximab remains effective in relapsed pts. The single agent ORR is ˜45%, but it’s more commonly combined w/chemotherapy
Very low-dose RT (400 cGy) may provide excellent local control & palliation in 70% of relapsed refractory pts
Radioimmunotherapy
Pts w/a short duration of remission may be considered for consolidation w/stem cell transplant after the 2nd– or subsequent-line Rx
Choice of autologous or allogeneic transplant is made individually; usu based on such factors as pt age & comorbidity, availability of a suitable donor, & the biology & behavior of the lymphoma
There is a ˜3%/y risk of transformation from FL to DLBCL, usu characterized by rapid nodal enlargement & rising LDH, + systemic sx
Bx required to diagnose transformation. PET may be useful in determining a site for bx. SUV values >10-13 suggest transformation (Ann Oncol 2009;20:508)
No intervention has been demonstrated to prevent transformation
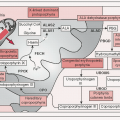
Thought to arise from chronic Ag stimulation due to infectious pathogens or other causes of inflammation, including autoimmune diseases
Variety of chromosomal translocations can result in Ag-independent activation of NF-κB pathway (important signal for B cell survival)
Whole genome sequencing of splenic MZL has identified recurrent somatic Mts of genes encoding chromatin-modifying enzymes & proteins in the NOTCH, NF-κB, & MYD88 pathways (JEM 2012;209:1537; NEJM 2012;367:826)
Comprise approximately 10% of all NHLs
MZL can affect diverse anatomical sites & presentation varies accordingly
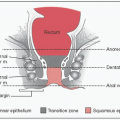
Localized sx: Epigastric pain (gastric), abdominal pain or bowel obstruction (intestine), cough (bronchial), orbital mass, salivary gland mass, thyroid mass, breast mass, skin nodules or rash, abdominal distension or early satiety due to splenomegaly (splenic MZL), LAN (nodal MZL)
| ||||||||||||||||||||||||||
Bx required: Either excisional/incisional or core plus FNA w/flow cytometry, splenic MZL often diagnosed on splenectomy specimen
Histology: Polymorphous infiltrate of small lymphocytes, centrocyte-like B cells, & plasma cells; reactive follicles; lymphoepithelial lesions (epithelial tissues)
Immunophenotype: CD19+, CD20+, CD22+, κ or λ restricted, surface Ig+, often cytoplasmic Ig+, CD5−, CD10−, CD23−/+, CD43−/+, CD103−, CyclinD1−
Cytogenetics/FISH: Most common t(11;18) (BIRC3:MALT1) → a/w H. pylori neg gastric ENMZL; t(1;14), t(14;18) (note this is IgH:MALT1), t(3;14); del13q; del7q
Molecular diagnostics for clonal IgH rearrangement (proves malignancy)
Lab evaluation: CBC w/diff, CMP, LDH, HBV sAg/cAb, HCV Ab, HCV PCR, HIV, SPEP/IFE (paraprotein often present), quant immunoglobulins
BM aspirate & core bx: Send for flow cytometry, splenic MZL classically shows “intrasinusoidal” lymphocytic infiltrations
Imaging: CT C/A/P w/contrast; may need MRI orbits (ocular) or neck (salivary); FDG PET not routinely required
Special considerations for gastric ENMZL:
→ H. pylori eradication w/abx for gastric ENMZL (not effective if translocations present or if there is muscularis or perigastric LN involvement)
→ Doxycycline for ocular or cutaneous ENMZL
Localized disease is common in MZL, so RT is an important tx modality (usu IFRT = involved field RT)
Localized disease is potentially curable so treat even asx pts
Adv stage MZL is a chronic disease (not curable), therefore decision to treat is based on presence of 1 or more GELF criteria (same as follicular lymphoma): ≥3 nodal sites each ≥3 cm in size, any involved site ≥7 cm in size, B sx, splenomegaly, pleural effusion or ascites, cytopenias (WBC <1, Plt <100), malignant cells in blood (>5000)
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Vaccinations: Pneumococcal & meningococcal 2 wks prior to splenectomy
Endoscopy: Surveillance EGD w/biopsies for pts w/gastric ENMZL treated by H. pylori eradication, not necessary after IFRT (Ann Oncol 2009;20 Suppl 4:113)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree