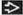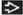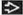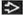ISCL/EORTC Revision to the Classification of Mycosis fungoides and Sézary Syndrome | From Olsen E et al: Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 110(6):1713-22, 2007. | |
TNM | Definitions | |
Skin | ||
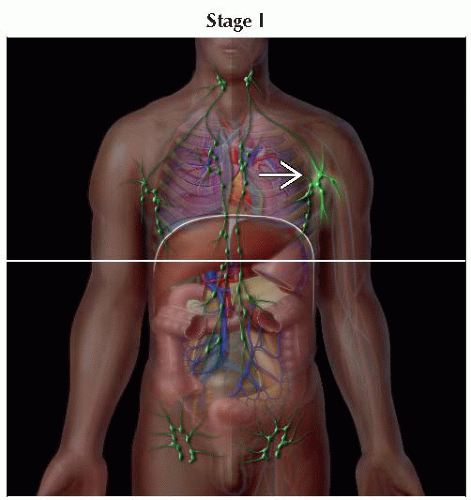
T1 | Limited patches1, papules, &/or plaques2 covering < 10% of the skin surface; may further stratify into T1a (patch only) vs. T1b (plaque ± patch) | |
T2 | Patches, papules, or plaques covering ≥ 10% of the skin surface; may further stratify into T2a (patch only) vs. T2b (plaque ± patch) | |
T3 | 1 or more tumors3 (≥ 1 cm diameter) | |
T4 | Confluence of erythema covering ≥ 80% of body surface area | |
Node | ||
N0 | No clinically abnormal peripheral lymph nodes4; biopsy not required | |
N1 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 1 or NCI LN0-2 | |
N1a | Clone negative5 | |
N1b | Clone positive5 | |
N2 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 2 or NCI LN3 | |
N2a | Clone negative5 | |
N2b | Clone positive5 | |
N3 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grades 3-4 or NCI LN4; clone positive or negative | |
NX | Clinically abnormal peripheral lymph nodes; no histologic confirmation | |
Viscera | ||
M0 | No visceral organ involvement | |
M1 | Visceral involvement (must have pathology confirmation6, and organ involved should be specified) | |
Peripheral Blood Involvement | ||
B0 | Absence of significant blood involvement: ≤ 5% of peripheral blood lymphocytes are atypical (Sézary) cells7 | |
B0a | Clone negative5 | |
B0b | Clone positive5 | |
B1 | Low blood tumor burden: > 5% of peripheral blood lymphocytes are atypical (Sézary) cells but does not meet the criteria of B2 | |
B1a | Clone negative5 | |
B1b | Clone positive5 | |
B2 | High blood tumor burden: ≥ 1,000/μL Sézary cells7 with positive clone5 | |
1 “Patch” indicates any size skin lesion without significant elevation or induration. Presence/absence of hypo/hyperpigmentation, scale, crusting, &/or poikiloderma should be noted. | ||
ISCL/EORTC Revision to the Staging of Mycosis fungoides and Sézary Syndrome | From Olsen E et al: Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 110(6):1713-22, 2007. | ||||
Stage | T | N | M | Peripheral Blood Involvement | |
IA | T1 | N0 | M0 | B0, B1 | |
IB | T2 | N0 | M0 | B0, B1 | |
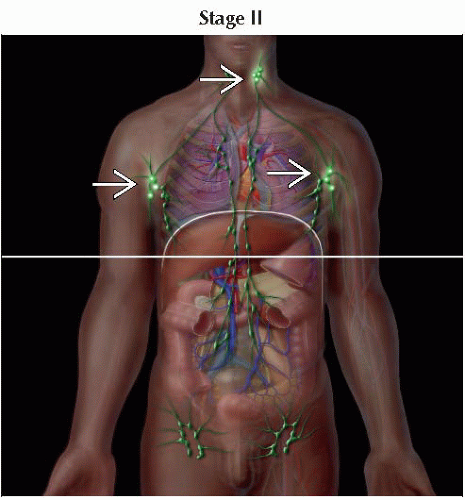
IIA | T1, T2 | N1, N2 | M0 | B0, B1 | |
IIB | T3 | N0-2 | M0 | B0, B1 | |
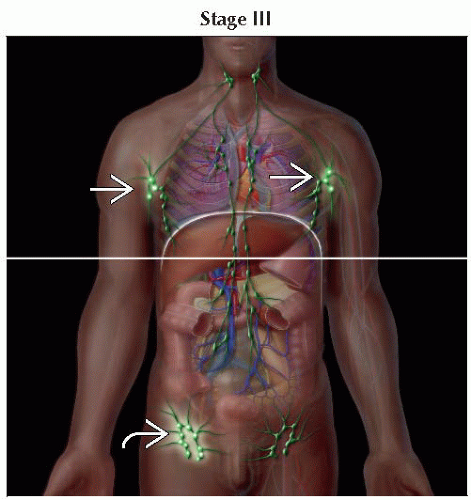
III | T4 | N0-2 | M0 | B0, B1 | |
IIIA | T4 | N0-2 | M0 | B0 | |
IIIB | T4 | N0-2 | M0 | B1 | |
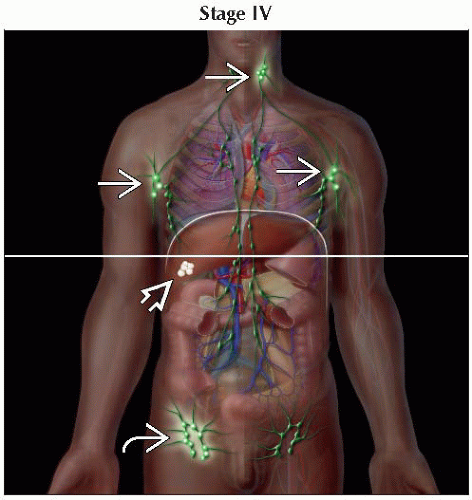
IVA1 | T1-4 | N0-2 | M0 | B2 | |
IVA2 | T1-4 | N3 | M0 | B0-2 | |
IVB | T1-4 | N0-3 | M1 | B0-2 | |
Histopathologic Staging of Lymph Nodes in Mycosis fungoides and Sézary Syndrome | From Olsen E et al: Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 110(6):1713-22, 2007. | |
Updated ISCL/EORTC Classification | Dutch System | NCI-VA Classification |
N1 | Grade 1: Dermatopathic lymphadenopathy (DL) | LN0: No atypical lymphocytes |
LN1: Occasional and isolated atypical lymphocytes (not arranged in clusters) | ||
LN2: Many atypical lymphocytes or in 3-6 cell clusters | ||
N2 | Grade 2: DL; early involvement by MF (presence of cerebriform nuclei > 7.5 μm) | LN3: Aggregates of atypical lymphocytes; nodal architecture preserved |
N3 | Grade 3: Partial effacement of LN architecture; many atypical cerebriform mononuclear cells (CMCs) | LN4: Partial/complete effacement of nodal architecture by atypical lymphocytes or frankly neoplastic cells |
Grade 4: Complete effacement | ||
St. Jude Staging System | From Murphy SB et al: Non-Hodgkin’ s lymphomas of childhood: An analysis of the histology, staging, and response to treatment of 338 cases at a single institution. J Clin Oncol. 7(2):186-93, 1989. | |
Stage | Definitions | |
I | A single tumor (extranodal) or single anatomic area (nodal), with the exclusion of mediastinum or abdomen | |
II | A single tumor (extranodal) with regional node involvement | |
≥ 2 nodal areas on the same side of the diaphragm | ||
2 single (extranodal) tumors with or without regional node involvement on the same side of the diaphragm | ||
A primary gastrointestinal tract tumor, usually in the ileocecal area, with or without involvement of associated mesenteric nodes only1 | ||
III | 2 single tumors (extranodal) on opposite sides of the diaphragm | |
≥ 2 nodal areas above and below the diaphragm | ||
All primary intrathoracic tumors (mediastinal, pleural, thymic) | ||
All extensive primary intraabdominal disease1 | ||
All paraspinal or epidural tumors, regardless of other tumor site(s) | ||
IV | Any of the above with initial central nervous system &/or bone marrow involvement2 | |
1 A distinction is made between apparently localized gastrointestinal tract lymphoma and more extensive intraabdominal disease because of their quite different patterns of survival after appropriate therapy. Stage II disease typically is limited to 1 segment of the gut ± the associated mesenteric nodes only and the primary tumor can be completely removed grossly by segmental excision. Stage III disease typically exhibits spread to paraaortic and retroperitoneal areas by implants and plaques in mesentery or peritoneum, or by direct infiltration of structures adjacent to the primary tumor. Ascites may be present, and complete resection of all gross tumor is not possible. | ||
Lymphoma: Neoplastic disease of lymphocytes with heterogeneous characteristics
Clonal expansion of lymphocytes
B cells
T cells
NK cells
Non-Hodgkin lymphoma (NHL) represents 85% of all malignant lymphomas
NHL, compared to Hodgkin lymphoma, is more likely to disseminate extranodally
Lymphoma broadly divided into 2 groups
Hodgkin lymphoma (HL)
NHL
Primary malignant tumors (WHO classification)
B-cell neoplasms
Peripheral (mature) B-cell neoplasms
Chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL)
B-cell prolymphocytic leukemia
Lymphoplasmacytic lymphoma
Splenic marginal zone lymphoma
Hairy cell leukemia
Plasmacytoma/multiple myeloma including solitary plasmacytoma of bone and extraosseous plasmacytoma
Extranodal marginal zone B-cell lymphoma, also called mucosa-associated lymphoid tissue (MALT) lymphoma (MALToma)
Nodal marginal zone B-cell lymphoma
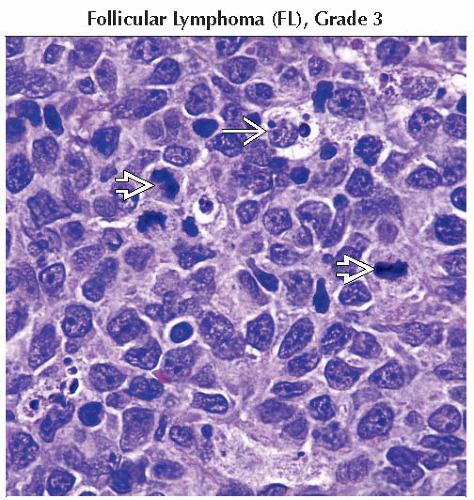
Follicular lymphoma, grades 1-3
Mantle cell lymphoma
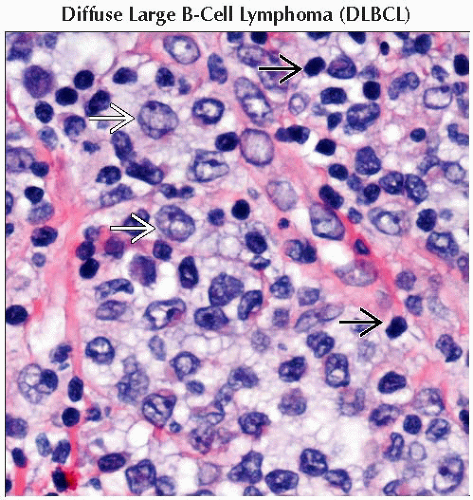
Diffuse large B-cell lymphoma (DLBCL)
Mediastinal (thymic) large cell lymphoma
Intravascular large B-cell lymphoma
Primary effusion lymphoma
Burkitt lymphoma/leukemia
Immunodeficiency-associated lymphoproliferative disorders
Associated with a primary disorder, HIV, methotrexate
Post transplant
Primary CNS lymphoma most often but not exclusively associated with AIDS
T-cell and NK-cell neoplasms
Precursor T-cell lymphoblastic leukemia/lymphoma
Peripheral (mature) T-cell neoplasms
T-cell prolymphocytic leukemia
T-cell large granular lymphocytic leukemia
Aggressive NK-cell leukemia
Adult T-cell leukemia/lymphoma
Extranodal NK-/T-cell lymphoma, nasal type
Enteropathy-type T-cell lymphoma
Hepatosplenic T-cell lymphoma
Blastic NK lymphoma
Subcutaneous panniculitis-like T-cell lymphoma
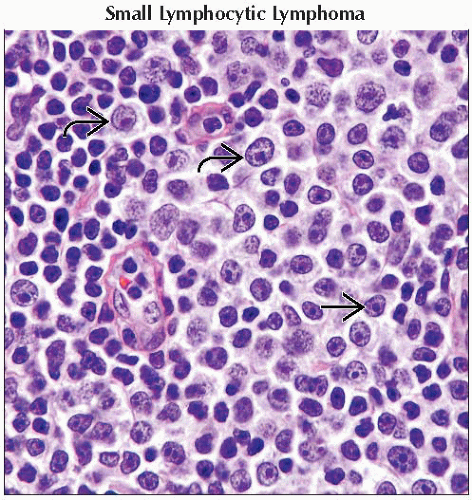
Mycosis fungoides/Sézary syndrome
Primary cutaneous anaplastic large cell lymphoma
Peripheral T-cell lymphoma, not otherwise specified (NOS)
Angioimmunoblastic T-cell lymphoma
Anaplastic large cell lymphoma
Hodgkin lymphoma, NOS
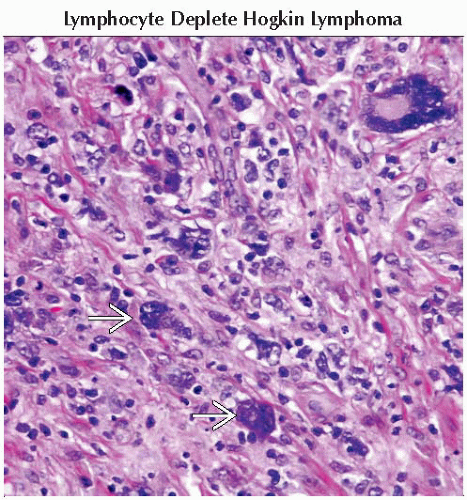
Nodular lymphocyte-predominant Hodgkin disease (NLPHD) (5%)
Classical Hodgkin lymphoma (95%)
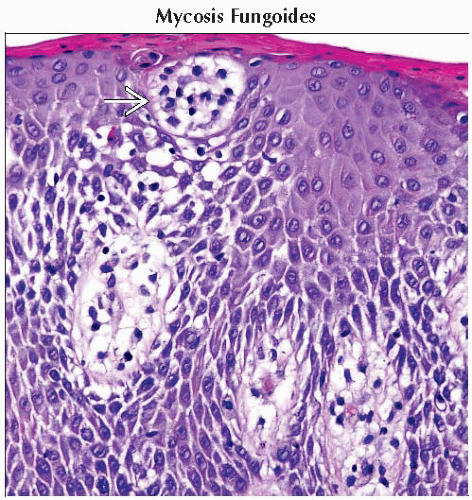
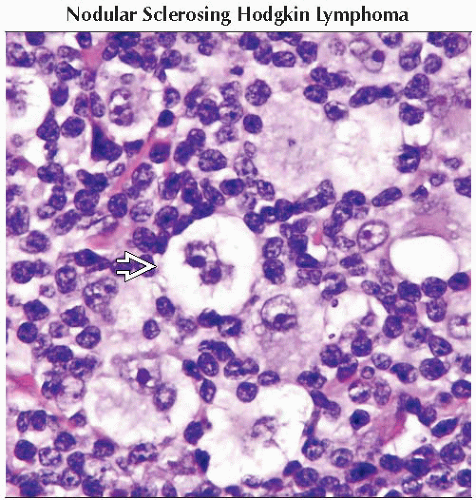
Nodular sclerosis (only type more common in women than men)
Lymphocyte rich
Lymphocyte depleted
Mixed cellularity
Comments
NHL
Diverse neoplasms with differing prognoses
B-cell lymphomas (90%)
T-cell lymphomas (10%)
More often involving extranodal sites than HL
Hodgkin lymphoma
Reed-Sternberg (RS) cells comprise minority of the tumor mass
Contiguous nodal involvement in central sites is most common
Location
B-cell lymphomas arise from the lymph node follicles
T-cell lymphomas arise in the paratrabecular region of lymph nodes
Risk factors
Immunodeficiency states
Epstein-Barr virus (EBV) is associated with African Burkitt lymphoma and some AIDS-associated lymphomas
Human T-lymphotropic virus type 1 (HTLV-1) associated with aggressive T-cell leukemia/lymphoma
H. pylori associated with gastric MALTomas
Chlamydia psittaci associated with orbital MALTomas
Number of cases in USA per year
NHL
70,130 estimated new cases of NHL with 18,940 deaths in 2012
7th highest incident cancer in men and women
9th cause of cancer death in men and 7th cause of cancer death in women
Probability of NHL from birth to death
Males: 1 in 43
Females: 1 in 51
Sex predilection
Males > females
Age of onset
NHL median age 67; HL has bimodal peak at 30 and 70 years of age
Common translocations
t(14;18), t(11;14), and some t(8;14) probably occur in primitive B cells in the marrow
Immunoglobulin gene rearrangements occur in B-cell neoplasms
T-cell receptor gene rearrangements occur in T-cell neoplasms
DLBCL
BCL6 mutations, t(14;18), and others
Follicular lymphomas
t(14;18) causes dysregulation of BCL2 and blocks apoptosis
Mantle cell lymphoma
BCL1 mutations, t(11;14)
Lymphoplasmacytic lymphoma
PAX5/IgH mutations, t(9;14)
Marginal zone lymphoma
AP12/MLT mutations, t(11;18)
Burkitt lymphoma
c-MYC translocations, t(8;n)
Anaplastic large cell lymphoma
NPM/ALK mutations, t(2;5)
NHL: 8% of patients have an autoimmune disease
HL: 9% of patients have or develop an autoimmune disease
Most common are Sjögren syndrome, thyroiditis, polymyositis, scleroderma, and glomerulonephritis
Paraneoplastic neurologic manifestations in HL include subacute cerebellar degeneration, limbic encephalitis, subacute necrotic myelopathy, and subacute motor neuropathy
Heterogeneous gross pathologies vary among subtypes of NHL
Hepatosplenomegaly depending on histology
H&E
Histological findings are heterogeneous in NHL, with morphology varying among subtypes
Both low- and high-power views are important to assess nodal architecture
Special stains
Immunohistochemistry is essential: B- &/or T-cell markers may be expressed depending on subtype
Flow cytometry often valuable
Cytogenetics often valuable
Molecular studies growing in importance
Local spread
Large mediastinal masses may invade chest wall, pericardium, or other thoracic structures
Lymphatic extension
NHL typically spreads to noncontiguous lymph nodes, leading to widely disseminated pattern of nodal involvement
Stages III and IV more common than stages I and II
NHL commonly spreads to extranodal sites
Hodgkin lymphoma typically spreads to contiguous lymph nodes, leading to pattern of nodal spread within same region
Stages I and II more common than stages III and IV
Rarely spreads to extranodal sites
Metastatic sites
Common sites include
Spleen
Liver
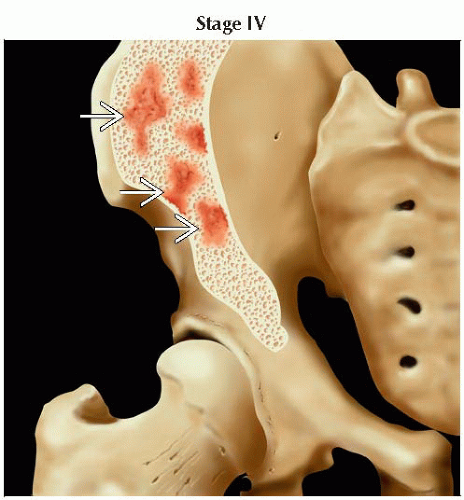
Bone
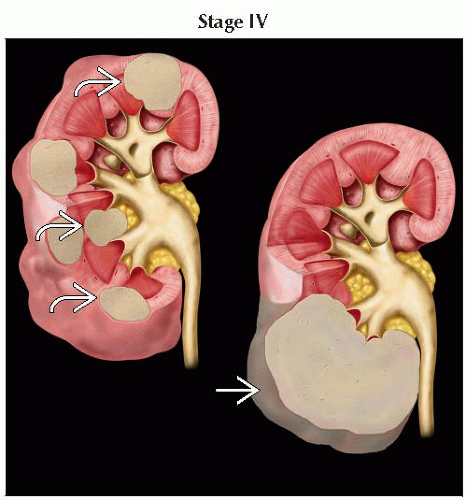
Kidney
General
Choice of modality often depends on specific factors, such as anatomic location
Often palpable abnormality, such as lymphadenopathy, may be initially evaluated with CT
Lymphoma is frequently a systemic disease; patients are routinely evaluated with CT or PET/CT
Initial evaluation most often includes axial coverage from skull base through pelvis
Ultrasound
Useful modality in initial evaluation of NHL for directing/guiding interventional diagnostic procedures
Evaluation of anatomic sites located superficially (i.e., neck, breast, axilla, extremities)
Will often see multiple, bilateral, nonnecrotic enlarged nodes
Despite large lymph node size, cystic necrosis is uncommon
Can also be used in assessment of spleen and kidneys
Right kidney may be imaged better than left kidney
Radiograph
Fairly limited in overall assessment with some exceptions, such as pulmonary NHL
Cavitating lesions may mimic tuberculosis
Because radiographic features are nonspecific, can be confused for variety of other processes, particularly infectious etiologies
Mammography
Can detect primary or metastatic NHL of breast
Primary lymphoma of breast is uncommon
Breast involvement appears as solitary mass without calcification
May also present with multiple masses, which makes differentiation of primary disease from metastatic disease impossible
Margins may be distinct or indistinct
CT
NECT
Cannot distinguish etiology of enlarged lymph nodes on NECT and CECT
Can detect diffusely enlarged spleen but may miss focal lesions without contrast enhancement
CECT
Optimal modality for detection in addition to FDG PET (PET/CT)
Will more accurately depict lesions that may be missed on NECT, particularly when there is organ involvement
Typically, portal venous phase of imaging is sufficient
Primary modality for overall evaluation of lymphoma
Primary head and neck extranodal lymphoma
NHL imaging findings may be identical to squamous cell carcinoma of pharyngeal mucosal space
Can arise from tonsils, mandible, hard palate, nasopharynx, parotid glands, nasal cavity, paranasal sinuses, pharynx, larynx, thyroid gland, and ocular adnexa
Most common sites of occurrence in pharyngeal mucosal space: Faucial (palatine) tonsil > nasopharyngeal adenoids > lingual tonsil (i.e., Waldeyer ring)
Accounts for up to 20% of all NHL cases
Thoracic lymphoma
CT and radiographs are primary modalities for evaluation of chest involvement
Radiologically, appearance of intrathoracic involvement in NHL may be similar to that of HL
Unlike HL, NHL is relatively evenly distributed in all mediastinal compartments, and posterior mediastinal lymphadenopathy is relatively common
CT can be used to evaluate for possible superior vena cava syndrome
CT is also used to evaluate abdomen, pelvis, genitourinary tract, and gastrointestinal tract
MR
Indicated for CNS imaging
Otherwise used as problem-solving tool
PET/CT
Can be used for initial diagnosis or detection of lymphoma
For example, in patients with retroperitoneal adenopathy not easily accessible by percutaneous means
PET may be falsely negative for some cell types such as MALT, mantle cell lymphoma, and small lymphocytic lymphoma
Optimally performed with contrast-enhanced CT as part of exam
Increasingly important as an early response indicator to therapy
Nuclear medicine
In general, PET/CT has replaced gallium-67 (Ga-67) for evaluating patients with lymphoma
Ga-67 sensitivity
Higher for more common histologic subtypes of low-grade NHL than for rare types
About equivalent for HL and high-grade NHL
Image-guided FNA
Often CT- or ultrasound (US)-guided
With sufficient sample, sensitivity and specificity > 90-95% may be achieved
In certain diagnostic settings, core biopsy or open resection may be preferred to evaluate nodal architecture
Residual masses
Open procedures may be preferred over percutaneous procedure
Fibrotic tissue increases likelihood of false negatives
Nodal disease
Ultrasound
Not often used for primary staging
Abnormal architecture in clinically suspected lymphadenopathy can establish presence of metastasis
US can be used to direct tissue sampling
CT
CECT used in detection of mediastinal nodes
NECT also capable of detection, but contrast facilitates measurement
Slice thickness between 5 and 10 mm generally sufficient to visualize chest nodes
Slight to moderate uniform enhancement following IV contrast, marked enhancement unusual (low attenuation in 20% of cases)
Calcification in affected lymph nodes prior to treatment is rarely seen
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree