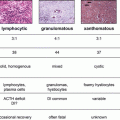
Ref
Testosterone, ng/dL (nmol/L)
SHBG (nmol/L)
LH (mIU/mL)
Estradiol, pg/mL (pmol/L)
Baseline
Post
Baseline
Post
Baseline
Post
Baseline
Post
1
13.4 ± 1.8
+58 %*
4.9 ± 0.8
+8 %
2
340 ± 21
+100 %*
39 ± 3
−21 %*
3
340 ± 130
+106 %*
4.8 ± 1.7
+21 %
48 ± 15
−28 %
4
256 ± 120
+100 %*
25 ± 12
+92 %*
3.69 ± 1.87
+5 %
28 ± 22
+21 %
5
8.75
+58 %
19.2
+100 %
2.31
+53 %
139.5
−14 %
There is some evidence that obesity disrupts spermatogenesis and predisposes to infertility [4]. A survey of American couples in Iowa and North Carolina revealed a twofold increase in infertility among men with BMI > 32 kg/m2 after adjusting for age and female BMI. In another study, BMI was lower in a cohort of fathers than in men attending an infertility clinic, but fathers were also slightly older. There are reports of an inverse association between BMI and sperm concentration, motility and/or morphology, but other studies have not confirmed these findings. Several studies have found lower inhibin-B levels among obese men, and one idea is that reduced proliferation of Sertoli cells in obese adolescents results in lower sperm counts in adulthood [5]. Fertility may also be influenced by decreased libido and erectile dysfunction. Obese men are usually fertile, however, so obesity may be an additive factor when other causes of infertility are present.
Treatment Strategies
Testosterone is often prescribed for men with symptomatic late onset hypogonadism because treatment usually increases mood and other quality-of-life measures [6]. Testosterone deficiency produced by GnRH analogs leads to a decrease in muscle mass and an increase in body fat. Thus testosterone deficiency may also be both a cause and a consequence of obesity and metabolic syndrome. Testosterone treatment tends to restore or to prevent the age-related decline in fat-free mass, reduces visceral adipose tissue, and improve insulin sensitivity. However, the changes are relative small in magnitude [7]. Testosterone treatment also tends to decrease both high-density lipoprotein and low-density lipoprotein cholesterol levels, but changes are again small and dose-dependent. Large doses of testosterone given to elderly men may cause edema and even contribute to congestive heart failure [8]. Testosterone stimulates erythropoiesis, and polycythemia, with its increased risk for stroke, may occur, especially in men with chronic lung disease treated with parenteral testosterone preparations.
Saad et al. [9] published a meta-analysis of the metabolic effects of various testosterone treatments on body composition in men with T2DM. They included 10 placebo-controlled studies of at least 6 months duration in which the increase in lean body mass was 2.49 ± 1.64 kg (range 0.8–6.2 kg) and the decrease in total fat mass was −1.47 ± 1.55 kg (range −0.6 to 4.8 kg). Thus the results show a consistent benefit, and interestingly a reanalysis of their data reveals a strong correlation between the changes in these two variables among the studies, with men receiving testosterone by the parenteral route experiencing the greatest change implying a dose effect (Fig. 36.1). Insulin resistance may improve, and there are small reductions in HbA1C in some but not all studies. Thus the current evidence indicates that testosterone treatment positively affects some metabolic parameters in men with metabolic syndrome or type 2 diabetes but how these changes influence clinical cardiovascular disease remains controversial.
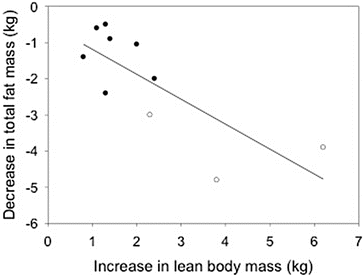

Fig. 36.1
Relationship between the increase in lean body mass and the decrease in total body fat observed in 10 studies of hypogonadal men with T2DM treated with a variety of testosterone preparations for at least 6 months. Open circles are from studies in which men received testosterone enanthate, mixed esters, or injected testosterone undecanoate; the closed circles are from studies in which men were treated with a transdermal testosterone patch (n = 3), a testosterone gel (n = 2), or oral testosterone undecanoate (n = 2). Data from Saad et al. [9]
Several recent studies have shown that men treated with testosterone are at increased risk for CVD. The first was a retrospective analysis of older men who had undergone coronary angiography at several Veterans Administration medical centers. Men subsequently prescribed testosterone (14 % of the total) had a 30 % greater likelihood of death, compared to untreated men. A second report was a respective analysis of a U.S. commercial and Medicare insurance database in which the authors assessed the diagnosis of acute MI within 90 days of filling a first prescription for a testosterone product (n = 55,593) or a phosphodiesterase type 5 inhibitor (n = 167,279). Although the number of events was small, testosterone-treated men > 65 years, and those below age 65 with a history of heart disease, had double the risk of heart attack within the 90 day window. These findings, although inconclusive, mandate the correct diagnosis of testosterone deficiency before recommending testosterone treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree