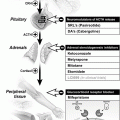
Fig. 6.1
Pituitary macroadenoma (arrows) abutting the optic chiasm superiorly on contrast-enhanced coronal (a) and sagital (b) MRIs
Other evaluations during his hospitalization revealed evidence of cardiomyopathy that was attributed to chronic tachycardia, and he underwent a successful ablation procedure restoring sinus rhythm. He was discharged home on carvedilol 12.5 mg twice daily, methimazole 20 mg three times daily, ramipril 2.5 mg daily, zolpidem and alprazolam as needed.
One month later, he presented to the endocrinology clinic with a TSH of 7.5 mIU/L, FT4 1.7 ng/dL, and total T3 206 ng/dL. Total testosterone was 581 ng/dL (250–1,100) and free testosterone was 52.3 pg/mL (35–155). The methimazole dose was increased to 30 mg three times daily and he was referred to us for further management and surgical intervention.
On his first presentation to our center, the patient described improvement in his thyrotoxic symptoms after 2 months of treatment with methimazole and beta blockers. He denied headaches, peripheral vision disturbances, weight loss, tremors, excessive sweating, or hyperdefecation. He also denied polyuria and polydipsia, change in shoe or ring size, or coarsening of his facial features. His libido remained intact and he denied galactorrhea or gynecomastia.
His past history was significant for multiple sclerosis and psoriasis, in addition to the pituitary adenoma. He had no family history of pituitary disease, but there was suspicion of thyroid disease in his father.
On examination his blood pressure was 126/72, heart rate 80 beats per minute and in sinus rhythm, temperature 97.2 °C, and his weight was 200 pounds. There was no lid lag or exophthalmos. His thyroid gland was normal in size and there were no nodules. Chest was clear to auscultation. Cardiovascular exam showed normal first and second heart sounds with no murmurs. The abdominal exam was unremarkable. There was no peripheral edema or tremor. His cranial nerve exam was intact, along with normal power and reflexes in his extremities. Psoriatic lesions on the extensor surfaces of his lower extremities were noted but the skin examination was intact otherwise.
Laboratory studies performed at the time (2 months after his diagnosis and initiation of therapy) showed a TSH of 68.59 mIU/L with a free T4 of 0.5 ng/dL. A formal visual field exam was normal. His methimazole dose was cut from 90 mg/day to 40 mg/day, but TSH continued to rise, reaching >150 mIU/L with FT4 0.2 ng/dL 2 weeks later, so methimazole was stopped. A month off methimazole, TSH was 18 mIU/L, FT4 1.7 ng/dL, Total T3 214 ng/dL, and thus he was restarted on 10 mg daily. Two months later, TSH was 57.7 mIU/L and FT4 1.1 ng/dL. The patient underwent transsphenoidal adenomectomy and pathology confirmed a pituitary adenoma. The immunohistochemistry was negative for ACTH, but focally positive for GH and prolactin. TSH staining was not performed.
On the first day after surgery, his TSH fell to 6.47 mIU/L. One month after surgery, his TSH was 2.95 mIU/L, FT4 0.6 ng/dL, and total T3 58 ng/dL. Prolactin was 4.2 ng/dL and morning cortisol was 17.7 μg/dL. Two months after surgery, TSH was 2.0 mIU/L, FT4 1.1 ng/dL and total T3 83 ng/dL. Other pituitary axes were within normal limits, but his total testosterone was 204 ng/dL, and is currently being watched for possible spontaneous recovery.
How the Diagnosis Was Made
The lack of suppressible TSH despite escalating levothyroxine therapy is certainly unusual in primary hypothyroidism. Nevertheless, the patient did not manifest frankly elevated thyroid hormone levels until a few years after his “hypothyroidism” diagnosis. This peculiar situation may be explained by the well described variation in the biological activity of the secreted TSH molecules, where despite being measurably elevated by TSH immunoassays, they may not have enough biological activity to produce high levels of thyroid hormones in vivo (see below). It is likely that as the tumor grew over time, it was able to produce more bioactive TSH, leading to frank elevation in thyroid hormones, which eventually lead to the correct diagnosis 6 years later. Retrospectively, his negative thyroid antibodies at the time of the “hypothyroidism” diagnosis are supportive of the hypothesis that he did not have primary hypothyroidism, but rather the elevated serum TSH levels due to the TSH-producing adenoma. Unfortunately, he was lost to follow-up for many years to later present with thyrotoxic symptoms and atrial flutter.
At the time of his latest presentation, both serum TSH and thyroid hormone levels were elevated suggesting secondary hyperthyroidism. With the elevated alpha subunit and the MRI evidence of an enlarging macroadenoma, it was clear that this was a TSH-producing adenoma.
An important and characteristic feature of adenoma thyrotrophs is their relative insensitivity to the negative feedback of thyroid hormones, leading to the hypersecretion of TSH despite the hyperthyroidism that was seen in this case. However, there is still integrity of the negative feedback of thyroid hormone, evidenced by the sharp rise of serum TSH levels when the patient was rendered hypothyroid on high-dose methimazole therapy.
Lessons Learned
TSH-secreting pituitary adenomas are considered the least common of all types of pituitary adenomas, accounting for 0.6–1.5 % of them. Thyrotrophs, which represent 5 % of adenohypophyseal cells, originate from the same common progenitor cell that expresses Pit-1 transcription factor along with somatotrophs (growth hormone-secreting cells) and lactotrophs (prolactin-secreting cells). Therefore, TSH-producing adenomas cosecreting GH or PRL are seen in up to one fourth of patients [1].
The majority (75 %) of TSH-producing adenomas are macroadenomas (measuring >10 mm) at the time of diagnosis, with no gender difference. The mean age at presentation is 45 years, with a wide age range (8–84). Those tumors are almost always benign but can be locally invasive, especially in patients with non-intact thyroid glands [2].
Patients typically present with overt thyrotoxicosis and diffuse goiters mimicking Graves’ disease, while others present with manifestations of a mass effect related to the pituitary tumor, resulting in headaches, visual field compromise, and/or loss of other anterior pituitary functions. In most patients, thyrotoxic symptoms are mild to moderate in severity [1]. Severe cardiovascular symptoms like atrial fibrillation or decompensated heart failure are less commonly reported compared to primary thyrotoxicosis. Nevertheless, similar to our patient, atrial fibrillation had been reported as the presenting symptom [3].
In most patients, a latency of a few years separates the onset of thyrotoxic symptoms from the establishment of the correct diagnosis; 6 ± 2 years in patients with intact thyroid glands, and 12 ± 3 years in patients who underwent unnecessary thyroidectomy or radioactive iodine ablation due to the erroneous diagnosis of Graves’ disease [4]. This latency seems to be shorter (4 ± 6 years) in more recent large series [5]. About one-third of patients with TSH-producing adenoma undergo unnecessary thyroidectomy or thyroid ablation due to misdiagnosis [6].
On clinical examination, up to 93 % of patients have diffuse or multinodular goiters due to chronic thyrotropin stimulation [1]. Other features of Graves’ disease like exophthalmos, pretibial myxedema, and positive antithyroid autoantibodies are absent. Nevertheless, cases of TSH-producing adenomas in patients with autoimmune thyroid disease including Graves’ disease and Hashimoto’s thyroiditis have been reported, with the clue to diagnosis of the latter being the lack of suppressibility of serum TSH by escalating thyroxine hormone replacement doses [7].
Laboratory investigations in patients with TSH-producing adenomas typically show elevated thyroid hormone levels with elevated, inappropriately normal, or incompletely suppressed TSH level (range from 0.4 to 393 mIU/L) [2]. This wide range of serum TSH levels despite frankly elevated thyroid hormone levels has been attributed to variations of the biologic activity of the secreted TSH molecules. It is important to stress that about 30 % of patients with TSH-producing adenomas present with serum TSH level in the normal range, which highlights the importance of measuring serum FT4 level in all patients with suspected thyrotoxicosis (and all patients with known pituitary disease).
The picture can be less obvious in patients previously treated with thyroid radioablation or thyroidectomy, since they do not have elevated serum thyroid hormone levels. In such patients, TSH levels tend to be much higher than in patients with intact thyroid glands. This observation highlights a peculiar feature of the tumoral thyrotrophs: that they are relatively resistant to the suppressive effect of elevated thyroxine, but retain sensitivity to the lack of thyroid hormones.
Most TSH-secreting adenomas produce high levels of alpha-subunit and an alpha-subunit/TSH molar ratio greater than 1 is described in about 82 % of cases. This is not true, however, for microadenomas where normal serum levels of alpha-subunit is the rule [5]. Elevated alpha-subunit levels should be interpreted with care in post-menopausal women or men with primary hypogonadism, given that alpha-subunit levels are higher in these circumstances.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree