Fig. 21.1
Serous borderline tumor
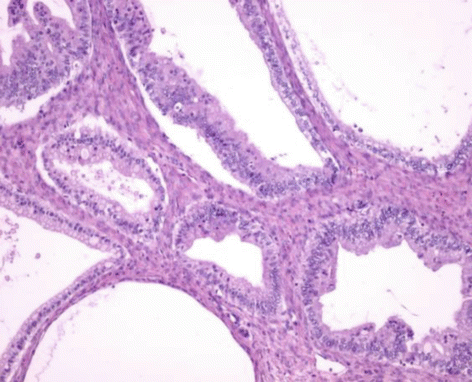
Fig. 21.2
Mucinous borderline tumor
A minority of serous borderline tumors (6–18 %) have an unusual micropapillary histological pattern (Fig. 21.3). A continuous 5 mm micropapillary growth pattern in a single slide is generally required for the diagnosis. They are more often bilateral, have a higher frequency of advanced stage, and, according to some authors, have a more aggressive course.
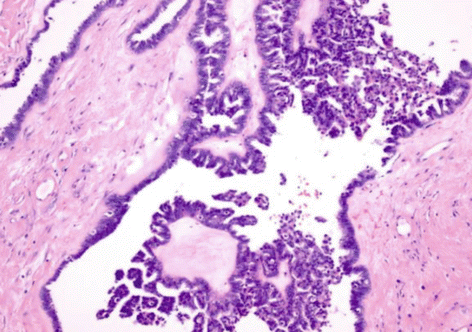

Fig. 21.3
Serous borderline tumor with micropapillary pattern
LMP tumors are staged as invasive ovarian cancer [8]. The documentation of a surface component or intraoperative rupture is important for an appropriate staging.
Peritoneal disease from borderline ovarian tumors is considered implants and not metastases, and they are distinguished as noninvasive (nearly 85 %) or invasive, according to the presence of stromal invasion. An important review reports a mortality rate for patients with noninvasive and invasive implants of 4.7 and 34 %, respectively [9], and for others the prognosis is similar.
Lymph node involvement at the time of surgery is rare, and few patients develop postoperative lymph nodal disease.
The risk of progression to invasive carcinoma is very low and estimated around 2–3 %.
21.3 Clinical Features and Diagnosis
Ovarian borderline tumors have the same clinical features of other pelvic masses. Woman can complain pain or distension of the abdominal circumference. More frequently, the mass can be completely asymptomatic and found during routine pelvic examination or abdominal ultrasound scan.
The relevance of serum tumor markers in patients with LMP tumors is controversial. Elevated serum concentrations of CA125 are reported in about 40 % of patients with stage I borderline ovarian tumors and in 83 % of women with advanced-stage disease. No data are available about other serum tumor markers (CA15.3, CA19.9, carcinoembryonic antigen).
Pelvic ultrasound is the gold standard for the definition of the pelvic masses, and many classifications have been proposed in literature [10].
Findings of radiological studies underline the contribution of perfusion-weighted and diffusion-weighted MRI sequences for differentiation between benign lesions, borderline ovarian tumors, and ovarian cancer [11].
Finally, the large majority of LMP tumors have been diagnosed incidentally intra- or postoperatively, and this issue represents a further complication in the management of this disease.
21.4 Treatment
Standard surgical treatment for LMP ovarian tumors includes bilateral salpingo-oophorectomy with or without hysterectomy.
In those patients with intraoperative diagnosis, staging surgery must be provided, and it consists in peritoneal washing, infracolic omentectomy, random peritoneal biopsies in at least six different areas of the abdomen, contralateral ovarian biopsy (if conservative surgery), and appendectomy in mucinous tumors [12]. The value of complete staging has not been demonstrated for early-stage cases, but the opposite ovary should be carefully evaluated for evidence of bilateral disease [13].
Patients with advanced disease should undergo a total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, node sampling, and aggressive cytoreductive surgery. Patients with stage III or IV disease with no gross residual tumor have had a 100 % survival rate in some series regardless of the follow-up duration [14].
In the last decade, because of the young age of most patients with LMP tumors and the highly favorable prognosis, conservative surgery that contemplates the preservation of the uterus and at least a part of one ovary is strongly increasing.
Several studies have been reported about conservative treatment in early-stage LMP ovarian tumors. Fertility–sparing surgery is associated with a higher rate of recurrences than radical surgery, but it does not affect on survival, because most recurrences are borderline lesions again and can be treated with secondary surgery, possibly conservative [15–17].
Conservative surgery can be either unilateral salpingo-oophorectomy or cystectomy. In case of cystectomy, the cyst is stripped off from the remaining ovarian parenchyma, and rupture or spillage inside the abdomen must be avoided. In case of laparoscopy, the specimen must be removed through an endo-bag.
In the literature, recurrence rates are between 0 and 20 % after unilateral salpingo-oophorectomy, between 12 and 58 % after cystectomy, and between 3 and 6 % after radical surgery [18]. The safety of conservative surgery for patients with stage I LMPT has been largely confirmed by many studies, and unilateral salpingo-oophorectomy must be considered as the first choice of conservative treatment; cystectomy should be counted for patients with a history of contralateral oophorectomy or in case of very young patients with bilateral tumor [16].
In case of fertility-sparing surgery, endometrial biopsy is recommended, because synchronous endometrial disorders (low-grade tumor or hyperplasia) may occur more frequently in young women.
After completion of childbearing, in patients with borderline tumors and in postmenopausal women, prophylactic contralateral salpingo-oophorectomy and hysterectomy are recommended.
The role of laparoscopy has been long discussed, because of the higher risk of cyst rupture and the potential risk of tumor metastasis in the port site [19]. Indeed, the frequency of cyst rupture during laparoscopy was the same as during laparotomy [20], and the rate of recurrence after laparoscopy is similar to conservative treatment. Few cases of port-site metastasis have been reported in literature after laparoscopy for borderline ovarian tumors, and none of them died of the disease.
In case of laparotomy, a vertical median incision is preferred to guarantee the access to the upper abdomen.
A very low rate (38 %) of adequate staging (peritoneal washing, peritoneal biopsy, and omentectomy) is reported in the literature during initial surgery, even when the borderline is intraoperatively diagnosed. In case of diagnosis with the final histology, every case should be discussed with a gynecologic oncologist. A surgical restaging does not seem to be necessary if the disease appears confined to a single ovary, because data suggest that complete staging does not improve the outcomes. However, the rate of recurrence is lower in those patients who received a complete staging than in those with incomplete staging [21]. According to available literature, surgical restaging could probably be omitted provided that the peritoneum is clearly reported as “normal,” there is no micropapillary pattern, and the patient accepts a stringent follow-up. On the opposite mucinous borderline ovarian tumors treated by cystectomy, those with micropapillary pattern and microinvasion possibly should undergo secondary restaging.
In advanced LMP tumors, surgery should include resection of all macroscopic peritoneal implants. Surgical procedures to achieve complete removal of peritoneal disease are the same as in advanced ovarian cancer, and postoperative residual disease is one of the most important prognostic factors [22]. In addition to the therapeutic effect, complete removal of peritoneal implants allows an accurate histological diagnosis.
Lymph nodes are involved in about 25 % of advanced [23], but several authors did not report any beneficial effect of the nodal dissection on survival in patients with borderline ovarian tumors [24]. In the absence of enlarged nodes or a frozen section suggestive of invasive implants, pelvic and para-aortic lymphadenectomy is not necessary.
The value of the second-look surgery in patients treated with conservative surgery has not been assessed in any study.
Adjuvant chemotherapy in women with early-stage serous borderline ovarian tumors does not have any beneficial effects on overall survival. In the same way, no advantages of adjuvant chemotherapy are recorded in patients with FIGO stage II–IV borderline ovarian tumors [25]. Debate persists for patients with invasive implants. Without any proven effectiveness, chemotherapy (platinum-based regimen with paclitaxel) is often proposed in patients with advanced LMP ovarian tumor with invasive implants. Some authors suggest the inhibition of PARP1 or MAPK pathway in specific settings.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree