24 Low-Grade Gliomas
Supratentorial infiltrative grade II glioma, as defined by the current World Health Organization (WHO) classification system (diffuse low-grade glioma [LGG]), is a complex and heterogeneous entity in adults, accounting for about 15% of all gliomas.1 Management of LGG patients has long been a matter of debate, for many reasons.
First, for a long time, the natural course of this disease was poorly studied. Indeed, in the classic literature, the vast majority of authors considered LGG as a “stable” and “benign” brain tumor. Therefore, the “wait and see” approach was advocated for many years, especially because LGG usually affects young adults (in the fourth decade of life) enjoying a normal life with no or only a mild deficit on a standard neurologic examination, even if they were taking antiepileptic drugs for seizures, as were 80 to 90% of patients.2
Second, it was traditionally thought that this infiltrative tumor cannot be removed without generating functional consequences, in particular when LGG is located in or close to so-called eloquent areas, as is commonly observed.
Third, mainly based on the subjective estimation of the extent of resection by the neurosurgeon, it was argued that surgical removal had no impact on the natural history of LGG. Thus, it was common to perform only a biopsy in order to obtain samples for neuropathological examination, and then to choose between a single follow-up or radiotherapy according to the morphological criteria established by the WHO classification (see below). Of note, the clinical results were evaluated in the majority of series on only few parameters, such as progression-free survival (PFS), overall survival (OS), and eventually Karnofsky Performance Scale (KPS) score.
Interestingly, recent technical and conceptual advances in genetics, cognitive neurosciences, imaging, and treatment revolutionized our knowledge of LGG, leading to the seminal principle of personalized management. Indeed, it is now well known that these aggressive tumors grow continuously, migrate along the white matter pathways, and inevitably progress to a higher grade of malignancy, leading to neurologic disability and ultimately to death. Furthermore, a better understanding of cerebral processing enables us to take into consideration interaction between the disease (the glioma) and the host (the brain), namely, mechanisms of neuroplasticity.3 As a result of this reorganization of cerebral networks, in parallel with developments in brain-mapping techniques, the benefit–risk ratio of surgery dramatically increased in the past decade. Such an improvement of the onco-functional balance of surgical resection should result in a paradigmatic shift in LGG, by switching from a traditional “wait and see” approach to an early and maximal resection based on functionalmapped guided resection. Moreover, a multistage surgical approach integrated in an individualized multimodal therapeutic strategy should be more systematically considered, with the ambitious aim of increasing the median survival as well as improving the quality of life (QoL)—that is, to solve the classic dilemma opposing OS against preservation of neurologic functions.4
In this setting, this chapter reviews the behavior and management of diffuse LGG in light of new insights provided by the recent literature.
 Pathology and Genetics: Toward a Molecular Classification of Low-Grade Glioma?
Pathology and Genetics: Toward a Molecular Classification of Low-Grade Glioma?
The WHO classification recognizes grade II astrocytomas, oligodendrogliomas, and oligoastrocytomas. Morphological features distinguish astrocytomas from oligodendrogliomas, but the diagnosis of oligoastrocytomas may pose difficulties and may be prone to neuropathologist subjectivity. Among WHO grade II astrocytomas, cellularity is moderately increased and nuclear atypia is occasional, but mitoses, endothelial proliferation, and necrosis are not present (although rare mitotic activity is permitted in a large specimen). Diffuse astrocytomas include fibrillary (the most common), gemistocytic, and protoplasmic variants. Of note, gemistocytic astrocytomas are more prone to malignant progression. The Ki-67/MIB-1 labeling index in diffuse astrocytomas usually is < 4%. The best immunohistochemical marker is glial fibrillary acidic protein, which is expressed in both tumor cells and astrocytic processes. In oligodendrogliomas, the nuclei are round and regular, and clear perinuclear haloes are present in most paraffin-embedded specimens (“fried-eggs”). They are moderately cellular and have a dense network of capillaries and frequently contain calcifications. Occasional mitoses and a Ki-67/MIB-1 labeling index up to 5% are compatible with WHO grade II oligodendrogliomas. There is no immunohistochemical marker specific for oligodendrogliomas.
Pitfall
• The World Health Organization classification for LGGs is based on criteria that are highly subjective to individual pathologist interpretation and not reproducible.
• It has been proposed that histological grading of low-grade glioma be replaced with a molecular and genetic classification system.
However, the WHO classification suffers from several limitations. First, it is not reproducible. This lack of reproducibility among pathologists is proven (or even when the same observer was asked to reinterpret the same histological preparations a few weeks later), with a difference of interpretation between reaction cells and tumor cells, and between astrocytes and oligodendrocytes. The interobserver discordance may reach 48% (recently reviewed elsewhere5). In addition, some elements are too subjective concerning the grading, such as the notion of anaplasia or cell density. Furthermore, the WHO classification does not distinguish tumoral cells from infiltrated residual brain parenchyma, and it considers the tumor to be homogeneous. Nonetheless, one frequently finds heterogeneous households on a background of diffuse LGG, corresponding to foci of increased cell density, possibly with cytonuclear atypical more pronounced than expected in LGG, which is why the term intermediate diffuse glioma was suggested for those cases in which the presence of these foci may lead to a faster evolution toward anaplasia.5
Advances in genetics brought new insights into the comprehension of LGG biology. The most frequent molecular alteration is the IDH1/2 mutation, which occurs at a very early stage and is reported in about 80% of LGGs. Thus, development of an IDH1-R132H mutation-specific antibody (H09) greatly assists in the diagnosis of astrocytomas, oligodendrogliomas, and oligoastrocytomas, and distinguishes these diffuse tumors from other lower grade gliomas. About 60% of diffuse astrocytomas carry TP53 mutations, which constitute a prognostic marker for shorter survival; gemistocytic astrocytomas carry TP53 mutations in > 80%, whereas combined 1p/19q deletion is rare. The molecular profile of oligodendrogliomas is the combined loss of 1p/19q occurring in 70 to 80% of these tumors, which is associated with longer survival, whereas TP53 mutations are encountered in only 5%. Most oligoastrocytomas carry either 1p/19q loss or TP53 mutations, with a tendency for these aberrations to be present in both tumor compartments.
Thus, since the vast majority (> 90%) of WHO grade II diffuse gliomas carry at least one of these alterations, the development of a molecular classification has been proposed that complements and eventually replaces histological typing. Interestingly, the molecular profile of LGG based on IDH1/2 mutations, TP53 mutations, and 1p/19q loss seems to provide a more objective classification that correlates well with patient survival.6 Additional genetic markers and molecular signatures have recently been proposed to refine the prognosis.7
 Clinical Presentation
Clinical Presentation
As mentioned, LGG usually affects young adults who enjoy a normal life. After an asymptomatic period that lasts several years (as demonstrated in incidentally found LGG), seizures are the most common presentation and may be partial or generalized. They occur in about 80 to 90% of patients and are intractable in 50%, especially in Rolandic, mediotemporal, and insular/paralimbic locations. Seizures are mainly due to cortical invasion, and are more frequently associated with oligodendroglial tumors. There is no clear association between severity of epilepsy and behavior of the tumor. Focal neurologic deficits due to mass effect, hemorrhage, and intracranial hypertension are not common presenting features. Indeed, neurologic deficits are rare, even if these tumors are frequently located within “eloquent areas,” because of cerebral plasticity mechanisms. This phenomenon is explained by the fact that LGG is a slow-growing tumor, giving the brain many years to accomplish functional remapping, with a recruitment of perilesional or remote areas within the ipsilesional hemisphere or of contrahemispheric homologous areas.8
Cognitive deficits, nonetheless, are often observed when objective neuropsychological assessments are performed at the time of diagnosis, despite a normal social and professional life, challenging the traditional view that LGG patients have a normal examination.9 Indeed, many LGG patients experience disorders of executive functions, attention, concentration, working memory, or emotion. These deficits can be attributed to the tumor itself, to seizures, and to antiepileptic drugs. Thus, a systematic assessment of higher functions and health-related QoL is now recommended before any oncological treatment for the following purposes: (1) to search for possible subtle neuropsychological deficits not identified by a classic neurologic examination; (2) to decide on a treatment strategy based on these individual results (e.g., decision of surgery first versus neoadjuvant chemotherapy in cases of very diffuse LGG-inducing important cognitive disturbances); (3) to adapt the surgical methodology to the results of this assessment (e.g., to perform functional mapping under local anesthesia even in the right hemisphere in right-handers in cases of preoperative language deficits, or to participate in the selection of optimal tasks to administer during awake surgery); (4) to provide a pretherapeutic baseline enabling a comparison with the posttherapeutic evaluation; and (5) to plan a specific functional rehabilitation following the surgical resection, which can induce a transient neurologic worsening.
Pearl
• Although patients with LGG are often considered to have normal neurologic functioning, neurpsychological evaluations often reveal subtle neurocognitive deficits, which should guide individual treatment plans.
 Imaging
Imaging
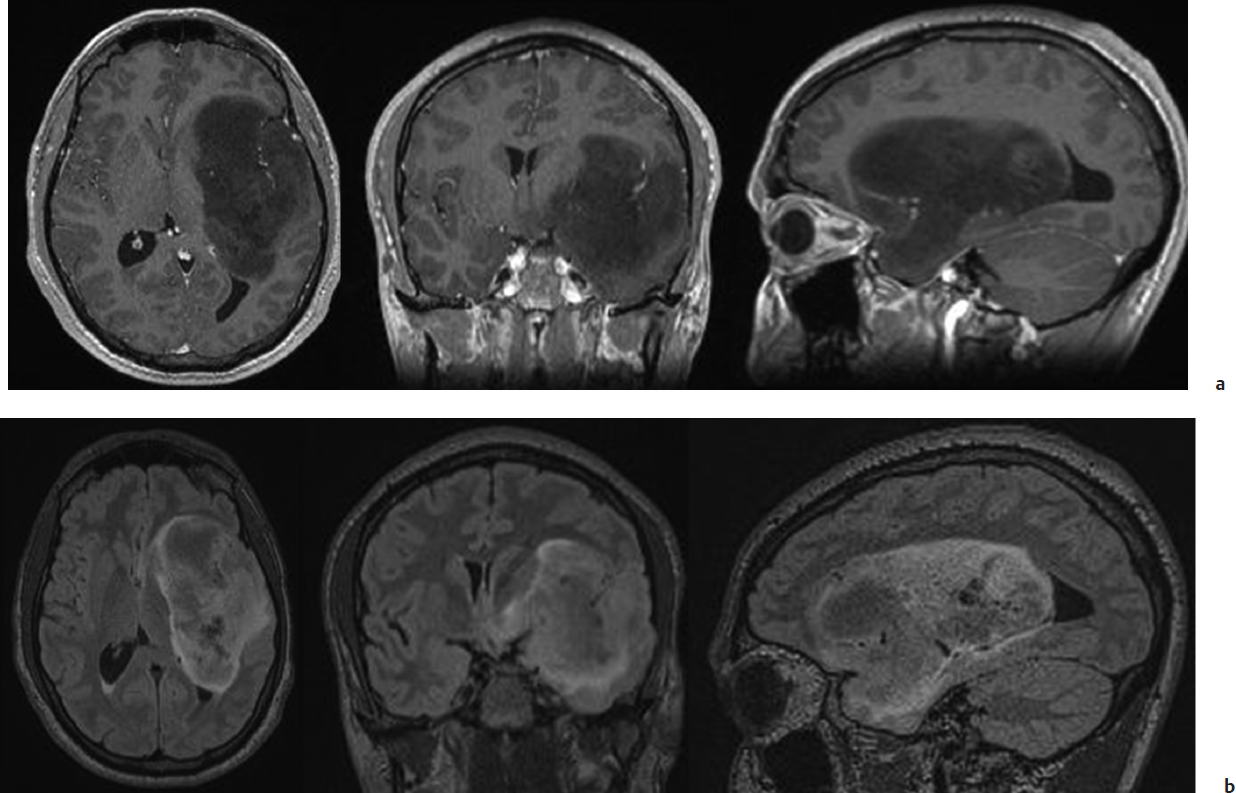
On magnetic resonance imaging (MRI), LGG is characteristically homogeneously isointense to hypointense on T1-weighted images and hyperintense on T2/fluid-attenuated inversion recovery (FLAIR)-weighted images (Fig. 24.1). When a nodular contrast enhancement is present, it indicates generally a focal area of malignant transformation, although patchy enhancement may remain stable over time in some tumors. However, one should be aware that this conventional structural MRI does not show the whole disease. Indeed, LGG invades the brain beyond the abnormalities visible on imaging, with tumoral cells present at a distance of 10 to 20 mm of the glioma boundaries defined by MRI.10
Recent advances in MRI sequence development and use provided a new conceptual approach to diagnosis and follow-up of LGG based on a multiparametrical and dynamic study of metabolism enabled by spectroscopy (even multinuclear) and perfusion-weighted imaging, namely, oncological biometabolic imaging. Proton magnetic resonance spectroscopy measures major metabolites in tumoral tissue. The typical (but not specific) spectrum of an LGG shows elevated choline, reflecting increased membrane turnover and decreased N-acetyl aspartate (reflecting neuronal loss). The presence of lactate and lipids is correlated to a more aggressive tumor.11 Dynamic susceptibility contrast MRI (DSC-MRI) enables the measurement of relative cerebral blood volume (rCBV), which is associated with vascularity. In astrocytoma, increase in rCBV in LGG predicts malignant transformation before contrast enhancement occurs. Diffusion-weighted imaging with calculation of apparent diffusion coefficient and positron emission tomography (PET) imaging (especially with 11C-methionine (MET) or 18F-fluoro-L-thymidine, which is a proliferation marker) can also be performed in LGG. Finally, metabolic imaging may be useful in guiding a biopsy to an area of high-grade activity.
Fig. 24.1a,b Typical magnetic resonance imaging (MRI) of a low-grade glioma (LGG), involving the left paralimbic system, with enhanced (a) T1-weighted and (b) fluid-attenuated inversion recovery (FLAIR)-weighted MRI in a patient enjoying a normal life.
• Functional MRI cannot differentiate essential regions from areas that can be functionally compensated (and thus removed).
From a functional point of view, advances in functional neuroimaging, for example, functional MRI (fMRI), magnetoencephalography, diffusion tensor imaging (DTI), and, more recently, transcranial magnetic stimulation, have enabled us to perform a noninvasive mapping of the whole brain. These techniques estimate the location of the eloquent areas (e.g., regions involved in sensorimotor, language, visual, and even higher cognitive functions) in relation to the glioma, and provide information with regard to the hemispheric language lateralization. Yet, it is crucial to emphasize that functional neuroimaging methods are not yet reliable enough at the individual scale, despite constant efforts for their improvement, mainly because they are based on biomathematical reconstruction; that is, their results may change according to the model used. Correlations with intraoperative electrophysiology have recently demonstrated that the sensitivity of fMRI is currently 59 to 100% for language (specificity, 0% to 97%).12
Diffusion tensor imaging enables the identification of the tractography of the main fiber bundles. This new method needs to be validated, especially by intraoperative electrophysiological techniques, before it can be used routinely for surgical planning. Indeed, comparison between distinct fiber tracking software tools found different results, showing that neurosurgeons have to be cautious about applying tractography results intraoperatively, especially when dealing with an abnormal or distorted fiber tract anatomy.13 Correlations between DTI and intrasurgical subcortical stimulation demonstrated that, despite a good correspondence in 82% of cases, DTI is not yet optimal to map language tracts in patients. Negative tractography does not rule out the persistence of a fiber tract, especially when invaded by a glioma.14 Moreover, DTI enables the study of the sole anatomy of the subcortical pathways, but not of their function.
 New Insights into the Natural Course of Low-Grade Glioma
New Insights into the Natural Course of Low-Grade Glioma
Contrary to what was claimed in the classic literature, there is no stable LGG. Objective calculation of growth rate (based on at least two MRIs spaced 3 months apart before any treatment) showed that all LGGs had a constant growth during their premalignant phase, with a linear increase of the mean diameter (computed from the volume) of about 4 mm/year.15 This growth was observed not only in symptomatic patients but also in incidentally discovered LGG.16 Thus, the concept of PFS is meaningless in LGG before any treatment or after an incomplete surgical resection because, in essence, all LGGs are continuously growing (whereas this end point would be unambiguous after a total resection or generally could be defined under adjuvant treatment such as chemotherapy/radiotherapy). In this context, the traditional imaging criteria, as initially proposed by MacDonald or more recently by the Response Assessment in Neuro-Oncology (RANO) group,17 are not appropriate to monitor LGG kinetics. Furthermore, there is an inverse correlation between growth rates and survival in LGG, showing that the mean velocity of diametric expansion is a better prognostic factor than the neuropathological examination performed according to the current WHO classification.15
These tumors are migrating along the white matter tracts (U fibers, association, projection, and commissural pathways). Thus, LGG is not a “tumor mass,” but is in fact an infiltrating chronic disease progressively invading the central nervous system, especially the subcortical connectivity. Such a diffusion of glioma cells may induce cognitive disorders, probably due (at least partly) to a “disconnection syndrome.”18
Finally, LGGs inescapably become malignant. Such malignant transformation leads to functional deficits, with a worsening of QoL, and ultimately to death. In two European Organization for Research on Treatment of Cancer (EORTC) randomized multicenter trials with more than 600 patients, in the subgroup of patients with a favorable prognostic score, the OS was 7.7 years, whereas in the subgroup of patients with a poor prognostic score, the OS was only 3.2 years.19 Recently, a study comparing early resection versus single biopsy demonstrated that the OS was 5.8 years in the biopsy group.20 Thus, these data show that LGG cannot be considered any more as a “benign” tumor but rather as a cancerous disease.
 Spontaneous Prognostic Factors
Spontaneous Prognostic Factors
A number of retrospective and a few prospective series have evaluated clinical, radiological, pathological, and molecular variables of potential prognostic significance in patients with LGG.19 Some of these factors have been fully validated. Clinically, age > 40 years, presence of neurologic deficits, absence of seizures at onset, and low performance status (KPS score < 70) are associated with a poorer outcome. Larger tumors, tumors crossing the midline, and rapid growth rate are also adverse prognostic factors. There are conflicting reports as to whether contrast enhancement is associated with a worse prognosis. A low CBV and low uptake of 11C-MET seem to correlate with longer OS. Histologically, oligodendrogliomas have a better prognosis than astrocytomas, whereas oligoastrocytomas have an intermediate outcome. Among molecular markers, 1p-19q co-deletion and IDH1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree