, Ahmad Ameri1 and Mona Malekzadeh2
(1)
Department of Clinical Oncology, Imam Hossein Educational Hospital, Shahid Beheshti University of Medical Sciences (SBMU), Shahid Madani Street, Tehran, Iran
(2)
Department of Radiotherapy and Oncology, Shohadaye Tajrish Educational Hospital Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran
A reduction in taste sensitivity (hypogeusia), an absence of taste sensation (ageusia), or a distortion of normal taste (dysgeusia) are well-known side effects in cancer patients that receive radiation therapy to the head and neck areas, and it has been reported in up to 100% of these patients [1–4]. The generic term of dysgeusia more commonly refers to any alteration in taste perception [5].
8.1 Mechanism
There are four basic taste intensities: salt, sour, bitter, and sweet. In addition to these basic tastes, a novel taste that is referred to by the Japanese word umami, which means delicious, has come to be recognized as a “fifth taste.” Umami is found in a diversity of foods (e.g., fish, meat, milk, tomato, and some vegetables), which is created by the combination of glutamate with 5′-ribonucleotides [6–8].
The sense of taste is mediated by the taste buds. They are found in the oral cavity, primarily on the dorsum of the tongue in the circumvallate, fungiform, and foliate papillae and on the palate, lips, cheeks, pharynx, epiglottis, larynx, and upper part of the esophagus [9, 10].
The taste bud is an onion-shaped epithelial structure with 50–100 tightly packed cells, including taste receptor cells, supporting cells, and basal cells [11]. Each taste bud opens to the epithelial surface via a small opening called the taste pore [12].
Taste receptor cells have short microvilli, which emerge from the apical region (outer end) of the taste cells to a taste pit below the inner taste pore. Taste receptor sites are located on membranes of microvilli. The microvilli are the portion of the cell that is exposed to the oral cavity [13]. The pore enables molecules and ions taken into the mouth to reach the receptor cells inside [12].
The taste receptor cells contact with afferent sensory neurons at their inner ends.
A nutritional, or trophic, interaction (i.e., one cell emits a substance that a second cell needs to grow) between the nerve fibers and taste buds exists. The interruption of the nerve fibers results in the disappearance of the taste buds [12].
Each of the different tastes is perceived in an individual taste bud with specific receptor cells that respond to distinct chemical stimuli. Furthermore, the diverse sites of the tongue surface are sensitive to a distinctive taste. For example, the anterior (tip) tongue is most sensitive to sweet or salty stimuli, while the lateral edges and posterior aspects of the tongue respond predominantly to sour and bitter substances, respectively. However, there is a concept that each taste cell and each site of the tongue surface may be sensitive to more than one taste [14], and all four different tastes can be perceived in all areas where taste buds are located [15].
The mechanisms involved in taste loss during radiation therapy are complex. Functional loss of taste buds occurs following radiation therapy due to the taste-cell microvilli or their membrane injuries. Membrane damage causes interruption of the synaptic contacts, impairment of taste bud trophic function of the nerve, and taste bud atrophy [12, 16, 17]. Functional loss of taste buds precedes cell loss, which occurs later following radiation therapy [12, 18].
8.2 Timing
Alteration in taste is an early and rapid response to radiation and often precedes mucositis [20]. Increase in taste thresholds begins from treatment with as little as 2–4 Gy and rises exponentially with a cumulative dose of about 30 Gy (3 weeks), 2 Gy per fraction. Rate of loss then slows down as the patients’ acuity approaches nil at >30 Gy [18]. Subjective complaints of taste also are started early after the beginning of the treatment, approximately 1–2 weeks after the initiation of treatment [3, 21, 22]. Perception of bitter and acid flavors is more susceptible to impairment than perception of salty and sweet flavors [23].
The clinical impairment pattern of umami taste has been investigated. Umami taste declines during the third week after the start of radiation therapy and improves after treatment conclusion [6, 24].
Loss of taste is usually transient. Patients often experience normal or near-normal levels of taste within 1 year after radiation therapy, although it can sometimes take a few years, or even a residual reduction in taste acuity may permanently remain [20, 23, 25–32].
8.3 Risk Factors
The proportion of the tongue that is contained within the radiation fields and the irradiation dose delivered has a significant correlation with the taste loss [12, 15, 34, 35]. A correlation between the tongue area that irradiated and the taste sensation mostly lost has been observed [18].
The presence of saliva plays a significant role in the normal taste acuity by transport and solubility of gustatory stimulants and protection of the taste receptors. Saliva production may be reduced by radiation therapy and affects taste sensitivity [11, 13, 20, 26, 34, 36].
Other factors that have some influence on the incidence or severity of loss of taste caused by irradiation are malnutrition or specific vitamin or mineral deficiencies like zinc deficiency [37] and destruction of the taste buds by the tumor [9]. Head and neck surgery by a reduction of the total number of taste buds or around the chorda tympani or glossopharyngeal nerve [2, 38] can also affect acuity of taste. Chemotherapy drugs [12, 39], oral mucositis [40], and infection [5] can exacerbate radiation-induced dysgeusia. Systemic disease like liver and kidney disorders, endocrine disorders, diabetes mellitus, psychological disorders, and central nervous system disorders, smoking, and alcoholism can also decrease taste [2, 41].
8.4 Symptoms
Irradiation of the taste buds typically leads to partial (hypogeusia) or complete (ageusia) inability to taste or an abnormal taste (dysgeusia) [18]. These taste abnormalities lead to the decreased hedonic aspect of food intake, decreased appetite, and reduced nutrient intake, leading to anorexia and weight loss [2, 18, 21, 37, 42].
The nature of the taste sensation modifies the volume and character of saliva. Loss of taste and failure of adequate salivation may explain difficulty in swallowing reported by some affected patients [37].
8.5 Diagnosis
Loss of taste may be a subjective response, which patients may indicate a presence of any subjective awareness of it.
Subjective awareness of taste loss and the presence of any distress caused by taste impairment (e.g., decreased enjoyment of food and appetite) can be assessed by using taste questionnaires [2], and objective detection of taste loss and recognition of the thresholds for each taste quality can be determined in each patient by the increase in threshold above the upper limit of normal and the lowest concentration of a solute that the patient distinguishes as different from water, respectively [9, 21, 43–45].
8.6 Scoring
Common Toxicity Criteria for Adverse Effect (CTCAE), version 4.03, scoring system has just defined two grades for taste abnormalities [46] (Table 8.1).
Table 8.1
CTCAE. V4.03 for taste
Definition | |
|---|---|
Grade 1 | Altered taste but no change in diet |
Grade 2 | Altered taste with change in diet (e.g., oral supplements); noxious or unpleasant taste; loss of taste |
8.7 Prevention
Prevention of taste loss can be obtained by the modification of radiation therapy including normal tissue shielding or placement of these tissues outside the radiation field by means of field arrangements or repositioning prostheses (e.g., tongue depressor) [47] or advanced radiation therapy technique (e.g., intensity-modulated radiotherapy) [48, 49].
The effect of amifostine on taste loss is inconclusive. The administration of amifostine may produce reductions in the severity of taste loss. However, the total frequency of dysgeusia can be more frequent among patients given amifostine than among controls [50, 51]. Further studies are needed in this regard.
All actions for prevention of xerostomia may be effective in reducing radiation-induced taste loss [48] (see Chap. 7).
There are a lack of data and controversial results on the effects of zinc supplementation in prevention/treatment of taste alterations in cancer patients (see Sect. 8.8).
8.8 Management
In most instances, taste gradually returns to normal or near-normal levels within 1 year after radiation therapy. Because of this transitory aspect, there is usually no need for special treatment [25], but clinicians can help their patients by offering counseling to improve their distressing symptoms. Dietary counseling and patient education can result in improved nutrition status and prevent weight loss.
Patients should be instructed about methods to increase taste, including preparing foods with strong taste and creating an attractive presentation of foods. Patients should be encouraged to avoid the use of tobacco and/or alcohol and management of hyposalivation and poor oral hygiene.
The assessment for taste alterations includes a thorough dietary history, eating habits, current appetite and desire for food, and weight measuring for comparison to baseline [52]. Nutritional counseling estimates each patient’s current nutritional status, calculates increase in energy and protein requirements to overcome deficits, and provides the therapeutic diet based on personal eating patterns and preferences, which are adjusted to the individual’s needs. Dietary counseling corrects patient diets with appropriate manipulation and consideration of foods with appealing taste, color, and smell; oral nutritional supplements may be added to the diet only in patients with inadequate food intake for more than 5 days or BMI < 18.5 [53, 54].
In the setting of significant weight loss (>2% loss in 1 week), patients should be evaluated by a registered dietician [52]. A dietician provides an individualized and intensive dietary counseling based on standard nutrition protocol, the Medical Nutrition Therapy (Cancer/Radiation Oncology) protocol of the American Dietetic Association (ADA), to maintain and/or improve a patient’s energy and protein intake [55–57].
In general, 25–30 kilocalories per kilogram body weight per day and 1–1.5 g of protein per kilogram per day are appropriate for those of normal weight. For those that are hypermetabolic or need to gain weight, 30–35 kilocalories per kilogram or greater and 1.5–2.5 g of protein per kilogram may be necessary [58].
Energy requirements can be calculated using various formulas, which give more precise estimates of resting energy expenditure. The Harris-Benedict equation is practical and reliable for measuring metabolic rate. The equation is used to estimate the basal energy expenditure (BEE) based on weight, height, and age [59]. To estimate daily energy requirements, basal requirements were multiplied by a 1.5 activity factor [60].
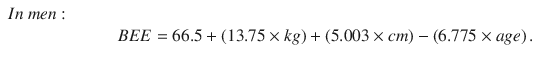
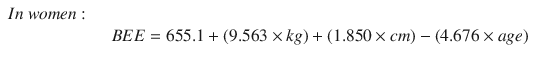
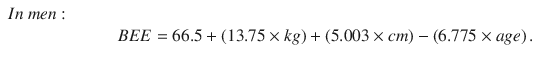
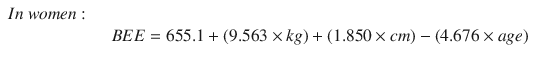
Zinc plays an important role in taste perception [61]. Zinc deficiency results in structural changes in taste buds cells, changes in the number and size profile of taste buds, and decrease in related nerve sensitivity [62, 63]. The effects of zinc supplementation in prevention/treatment of taste alterations in cancer patients are controversial. Some have found that the administration of zinc sulfate (45–50 mg orally three times daily) in cancer patients that had received radiation therapy to the head and neck region is an effective approach both in the prevention and correction of taste abnormalities [2, 3, 21, 64]. However, some found no statistically significant effect of zinc sulfate therapy on radiation-induced taste alterations [65]. Further studies with longer follow-ups and with different doses of zinc supplementation are needed in this regard.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree