Staging of rectal cancer is the process of determining the extent of tumor spread, and serves two related goals: to provide information on the prognosis and to guide treatment choices. With an increasing number of treatment options and the current trend for further personalizing treatment, accurate staging of rectal cancer is becoming highly relevant in making therapeutic decisions. Traditionally imaging was mainly used for assessing distant spread of rectal cancer to the liver or lungs at primary presentation (cM-staging) while locoregional extent of tumor was assessed through histological examination of the resection specimen (pTN-staging). This histological staging was then used to guide decisions on adjuvant systemic therapy to prevent systemic recurrence, and on adjuvant locoregional (chemo)radiation to prevent local recurrence. When (chemo)radiation was shown to be more effective in a neoadjuvant setting,1 the selection process for (chemo)radiation changed from a histology-based to an imaging-based risk assessment. In addition, the neoadjuvant therapy altered the histological postoperative assessment, as well as the selection process for adjuvant systemic therapy—which is increasingly predicated on the primary imaging-based risk assessment. The histological risk factors associated with local recurrence and distal recurrence are generally similar: T stage, N stage, circumferential resection margin (CRM), extramural venous invasion (EMVI), perineural invasion, lymph and blood vessel invasion, and histological grade.2,3 Although modern CT techniques are improving and are, to some extent, able to provide information for locoregional staging, endorectal ultrasonography (ERUS) and magnetic resonance imaging (MRI) are considered the two best locoregional staging methods, providing information on the rectal tumor and surrounding mesorectum (T and N stage, distance to the mesorectal fascia, EMVI). The histological cutoff points of the TNM staging system, such as the distinction between a T2 and T3 tumor, do not always easily transfer to staging through imaging, because of the lack of histological precision. The importance of this difference for a tumor that is on the borderline is, however, questionable, and imaging can provide other prognostic factors such as depth of ingrowth into the mesorectum.
After neoadjuvant chemoradiotherapy (CRT) there is often considerable downsizing and downstaging of the tumor, with 8% to 24% of patients obtaining a pathological complete response (pCR).4 Traditionally surgeons have based the extent of the resection on the pre-CRT imaging, mainly because of the fear of leaving behind small islands of viable tumor in the treatment-induced radiofibrosis. There is, however, an increasing awareness that the response to neoadjuvant CRT can be better exploited in order to perform a less extensive operation with less morbidity and a better functional outcome. In locally advanced tumors that respond very well, it may no longer be necessary to perform an extramesorectal resection, and in tumors that are very close to the sphincter it may become possible to perform a sphincter-saving total mesorectal excision (TME). A number of reports even suggest the options of local treatment, that is, transanal excision (TAE) of the small tumor remnant or scar, or a completely nonoperative approach for a clinical complete response (cCR).5,6 These approaches all involve a trade-off between the risk of suboptimal oncological control and the gain in function and decreased morbidity, a risk that can be minimized with accurate restaging techniques.
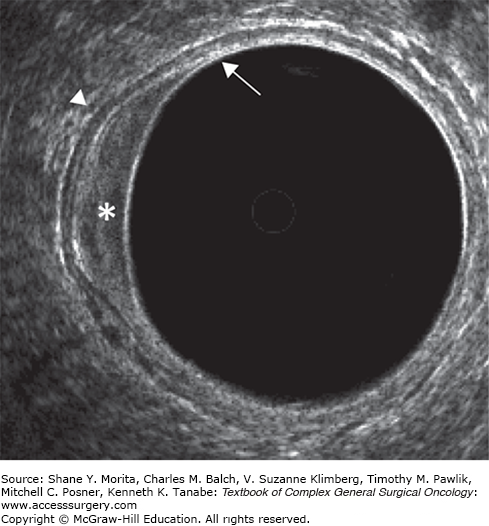
Endorectal ultrasonography is an established staging modality in patients with rectal tumors.7,8 A meta-analysis showed a pooled sensitivity and specificity for ERUS staging of 94% and 86% in the setting of tumor ingrowth into the bowel wall, 90% and 75% for perirectal tissue invasion, and 70% and 97% for organ invasion, respectively.9 The agreement between uT-stage and pT-stage in the larger studies is 65% to 70%, with 10% to 15% understaging and 20% overstaging.10–12 ERUS can discriminate between mucosal pT0 lesions and pT1 tumors, with a risk of understaging for uT0 of only 5% to 15%.12–16 In uT1 there is understaging in 15% to 20%, and in uT2 stage 15% to 30%, while overstaging in uT3 occurs in 25% to 30%. ERUS is accurate in assessing tumor invasion into organs such as the vagina, uterus, prostate, and seminal vesicles, especially in tumors that are within reach of the probe. ERUS is less suitable for evaluating tumor involvement of the mesorectal fascia (MRF), a structure that is not well recognized by ERUS. Moreover, ERUS provides only a limited view of the extent of tumor and its relation to the surrounding pelvic structures—especially in those areas outside the scope of the probe. Although ERUS is less suitable for depicting the exact tumor extent in larger tumors, it remains the method of choice for staging superficial cT1 rectal cancers because it is the only imaging technique that can visualize the individual layers of the bowel wall (Fig. 107-1).
FIGURE 107-1
Endorectal ultrasonography of a patient with cT1 rectal cancer. The tumor (white asterisk) invades the hyperechoic submucosal layer (white arrow), but does not invade the hypoechoic muscular bowel wall (white arrow head). ERUS visualizes all individual layers of the bowel wall. Therefore, ERUS remains the first method of choice for staging superficial (cT1) tumors.
Although CT is superior to ERUS and, because of its wider field of view, more accurately assesses tumor extension into the pelvic structures, CT is not as accurate as MRI because of its inferior contrast resolution. Bipat and coworkers pooled the data of CT studies published between 1985 and 2002, reporting a pooled sensitivity and specificity of 79% and 78 %, respectively, in the setting of perirectal tissue invasion, and 72% and 96 %, respectively, for organ invasion.9
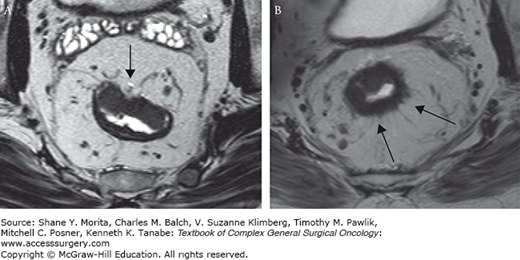
International guidelines increasingly incorporate MRI as part of the standard workup of patients with rectal cancer.17,18 A meta-analysis of MRI studies published between 1993 and 2002 reported a pooled sensitivity and specificity of 94% and 69%, respectively, in the staging of cT2; 82 and 76%, respectively, for cT3 and 74; and 96% for cT4 tumors.9 Most staging failures with MR imaging occurred in differentiating between T1 and T2 lesions, and T2 and T3 lesions. On MRI, a T1 tumor cannot be reliably distinguished from a T2 because the submucosal layer is generally not visualized. Like ERUS, MRI cannot distinguish between some borderline cT2-cT3 lesions, especially when there are spiculated desmoplastic extensions of the tumor in the surrounding mesorectal fat tissue (Fig. 107-2a, b). In these borderline lesions the exact T stage may, however, be clinically less relevant, as the depth of ingrowth into the mesorectum has been shown to be an independent prognostic variable, with an early T3 lesion behaving more like a T2 lesion.19 This has been confirmed in other studies, and a subdivision of T3 tumors—according to depth of tumor ingrowth into the mesorectum—has been recommended in the TNM Supplement of the International Union Against Cancer (UICC).20 A large European multicenter study showed that MRI is very accurate in predicting this extramural ingrowth, and can be used to select a prognostically good group of tumors with up to 5 mm of ingrowth.21,22
FIGURE 107-2
MR images of a male patient with pT2 rectal tumor (A) and a male patient with a pT3 rectal tumor (B). In both patients the dark bowel wall cannot be clearly delineated at the circumference of the tumor (black arrows). Instead subtle desmoplastic stranding is shown. The MR image of a pT2 rectal cancer with desmoplastic stranding into the mesorectal fat mimics that of a pT3a-b rectal tumor with desmoplastic reaction. Overstaging errors are known to occur in 30% to 40% of these borderline tumors.
The lateral or radial resection margin of a TME procedure is just outside the MRF. A positive CRM can thus be the result of inadequate TME surgery, or an advanced tumor that comes close to or invades the MRF. Preoperative identification of patients with a threatened or involved MRF identifies tumors at high risk for a local recurrence; these patients are generally treated with a long course of chemoradiation followed by a long interval prior to surgery to provide optimal tumor downsizing. It is also relevant for the surgeon to know the exact relation of the tumor to the surrounding pelvic structures and MRF in order to obtain a complete resection.
The role of helical multislice CT (MSCT) for the assessment of the MRF has been studied in a prospective multicenter study.23 For mid and high rectal tumors CT is reasonably good in ruling out an involved MRF with a sensitivity of 76% and a specificity of 96%. For low rectal cancer this is more difficult, because low in the pelvis the structures are close to each other with little or no interposed fatty tissue. CT has inherently less contrast resolution than MRI, which in these low tumors leads to a sensitivity of only 66% and a specificity of 82% for the involvement of the MRF.
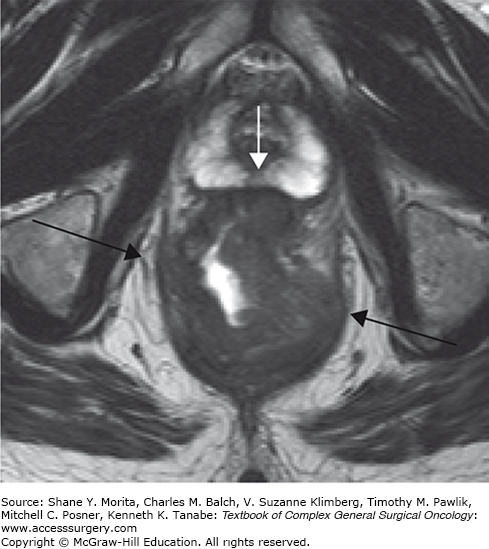
At present, because of the inherently high tissue contrast and high spatial resolution of modern MR equipment, MRI is the best imaging method for providing detailed information on the relation between tumor and surrounding structures. This detailed anatomical information can serve as a road map for surgery, and it is often said that, with MRI, “what you see is what you get” (Fig. 107-3).
FIGURE 107-3
MRI of a male patient with a locally advanced low rectal cancer. The relation between the tumor and surrounding normal structures is well appreciated on MRI because of the inherent high tissue contrast. Anteriorly the tumor is threatening the prostate (white arrow) and this organ is at risk for tumoral invasion. Laterally the tumor is invading the mesorectal resection margin and threatens the pelvic floor muscles (black arrows). MRI serves as an excellent road map before surgery. It is often said that with MRI “what you see is what you get.”
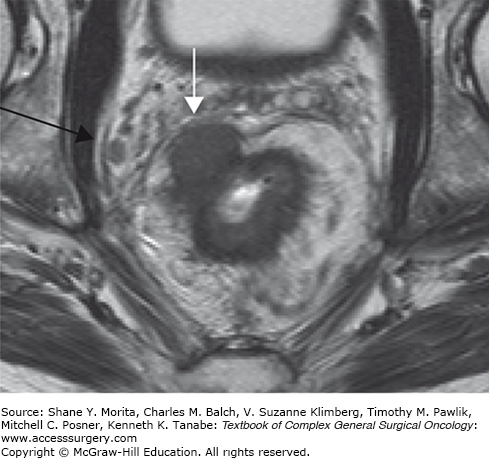
An involved MRF on MRI is defined as a closest distance of ≤1 mm between tumor and resection margin, as this represents the optimal prognostic cut-off point. Many single-center studies have shown that MRI is highly accurate in the assessment of an involved MRF (Fig. 107-4).24,25 The results of a systematic review confirm the high performance of MRI, showing a sensitivity in assessing an involved MRF of 60% to 88%, and a specificity of 73% to 100%.26 The subsequent European multicenter study showed a sensitivity of 59%, specificity of 92%, PPV of 54%, and NPV of 94%.27 When MR findings are incorporated into the multidisciplinary decision-making process of rectal cancer treatment, a decrease in the number of positive margins was found.28 A recently published multicenter cohort study showed that differentiated treatment of primary rectal cancer based on MRI can result in a proportion of complete resections as high as 96% (218 out of 228 included patients) and can reduce—at a median follow-up of 41 months—the 3-year local recurrence rate to as low as 2.2%.29
An important risk factor for both local and distant recurrence is the presence of lymph node metastases, and this is generally considered an indication for neoadjuvant radiotherapy.17 Identifying nodal disease with imaging remains difficult, however, because the use of size criteria alone as a measure of metastases results in only moderate accuracy. Lymph nodes with a diameter of >8 mm are invariably malignant. However, many of the involved lymph nodes in rectal cancer are smaller than 5 mm.30 Additional morphological criteria have been proposed, such as round shape, an irregular border, and heterogenous texture indicating malignancy (Fig. 107-5). In two meta-analyses, ERUS performed slightly better than CT or conventional MRI, most likely due to the additional inclusion of these morphological criteria in the ERUS studies.9,26 The pooled sensitivity and specificity for nodal involvement was 67% and 78% for ERUS, 55% and 74% for CT, and 66% and 76% for MRI. A more recent meta-analysis of MRI showed accuracy in the same range: sensitivity of 77% and specificity of 71%.25 An advantage of MRI over ERUS is the wider field of view, which can visualize nodes high in mesorectum, along the superior rectal vessels—an area beyond the scope of the endosonography probe. The moderate accuracy of nodal staging was illustrated in a study by Guillem et al reporting on a group of cT3N0 tumors, staged by ERUS (69%) or MRI (31%), that were subsequently treated with CRT and surgery:31 on histology, node-positive disease was observed in as many as 22% of the tumors!
FIGURE 107-5
MRI of a male patient with a low rectal cancer and suspicious mesorectal nodes. The tumor is shown in the low rectum. Because the dark muscular bowel wall is well delineated at the level of the tumor (black arrow), it is likely that the tumor is limited to the bowel wall (cT2). In the dorsal mesorectum at level S1–3 nodes are visualized with at least two out of three morphological MR features of malignancy: round shape, irregular border, and heterogenous texture (white arrows).
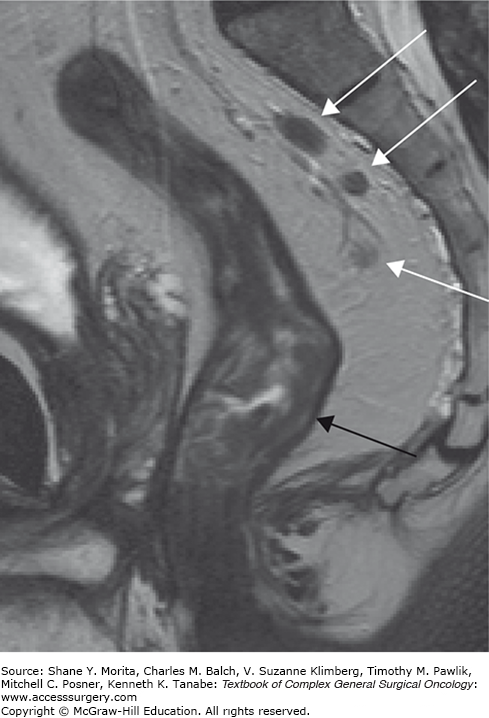
However, others have confirmed that the addition of morphological criteria to size can improve the accuracy of MRI in detecting nodal metastases.32 These morphological features are well appreciated if the nodes are large, but more difficult to define in smaller nodes, as these features may remain beyond the resolution of even high-resolution MR technology. A recent consensus meeting of 14 rectal cancer imaging specialists from the European Society of Gastrointestinal and Abdominal Radiology stated that although size thresholds of 5 to 8 mm were common in clinical practice, “no single diameter threshold is sufficiently accurate to differentiate benign from malignant nodes, and … the choice of a threshold is contingent upon the desired balance between sensitivity and specificity, which varies per clinical setting.”18 So, how does one work with this in clinical practice? When no nodes are visible on MRI, it is safe to call it N0 disease. When a node is larger than 8 mm, it should be considered malignant. For the smaller nodes our center uses the following (somewhat arbitrary) guideline: nodes of 5 to 8 mm are considered suspicious when at least two of the morphological criteria are present (round shape, irregular border, or heterogenous texture of the node); nodes <5 mm are deemed suspicious only when all three criteria are present. Although this strategy will miss some small involved nodes, it avoids overcalling and overtreating many node-negative tumors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree