Advances in surgical, radiation, and interventional radiology therapies carry a reduction in morbidity associated with therapy. Aggressive management of patients with oligometastatic non–small cell lung cancer offers the potential for improved disease-free survival and quality of life compared with traditional systemic therapy alone.
Key points
- •
Stereotactic ablative radiotherapy (SABR) carries the potential for durable local control of either solitary extrathoracic metastasis or persistent primary disease.
- •
Intracranial radiosurgery for small volume intracranial metastatic disease is the preferred first-line treatment at diagnosis for non–small cell lung cancer, with or without operative intervention.
- •
In absence of published randomized trials, the use of SABR in current clinical practice is most appropriately determined in the context of a multidisciplinary thoracic oncology team.
Introduction
From the very onset of an oncologist’s clinical and specialty training, the concept of routine adherence to evidence-based medicine and clinical practice guidelines molds the physician to prescribe and administer therapy based on primary cancer site and stage. The oncologist follows established national and institutional pathways while simultaneously synthesizing the balance of documented disease presentation, patient age, performance status, and medical comorbidities to create a plan of care. Treatment is dynamically modified to disease response or progression, provided the patient remains an appropriate candidate for additional management. Successful eradication of disease earns professional and personal satisfaction, whereas progression of disease inherently necessitates the reevaluation of the therapeutic approach.
The rapid evolution of nontraditional treatments in the field of oncology medicine has afforded new opportunities for consideration of the inclusion of therapeutic approaches for isolated cancer lesions that extend beyond accepted guidelines and pathways. Minimally invasive surgical approaches, whether robot assisted or laparoscopic, often reduce the expected risks and complications previously considered barriers to their inclusion as part of cancer treatment. The evolution in clinical experience regarding the technical capabilities, applications, and toxicities for ablative radiotherapy to address specific sites of active disease remains an active area of clinical research and investigation. Radiofrequency or cryotherapy ablations offer additional opportunities for focused tissue destruction to address isolated foci of malignancy.
The oligometastatic disease state was first defined in 1995 and refers to a stage of disease, where cancer has spread beyond the site of origin, but is not yet widely metastatic. In such a state of limited metastatic disease burden, it is hypothesized that eradication of all sites of metastatic disease could result in long-term survival, or even cure, in a subgroup of patients. Ablation of metastatic deposits can be achieved surgically, or through stereotactic ablative radiotherapy (SABR), a new radiotherapy technology that delivers very large, hypofractionated doses of radiotherapy with high precision to small tumor targets, with high rates of local control.
Clinical evidence to support the presence of an oligometastatic state is emerging in both the surgical and SABR literature, for several tumor types. In a study of more than 5200 patients with lung metastases who underwent surgical resection, a 5-year survival of 36% was reported in patients who achieved a complete resection, which is much greater than would be expected for many patients with disseminated stage IV disease. Similarly, in patients treated with SABR for 1 to 3 lung metastases from a variety of primary tumors types, local control with SABR was 96% at 2 years, and the 2-year survival was 39%. The potential for long-term survival has been demonstrated in patients treated for oligometastases using surgery or SABR for metastatic lesions located in the liver, brain, bone, and adrenal glands. However, the risk of new metastatic disease, in either a new site or increase in clinical volume, remains high after ablative treatment, with reported rates up to 60% to 80%. Although SABR may be used as a further salvage treatment, often patient performance status and socioeconomic factors create functional limitations in the routine application for cancer treatment.
Despite the achievement of long-term survivors beyond statistical mean survivals with ablative treatment for oligometastatic disease, the level of evidence to support such treatments in routine clinical practice is weak in many cases, often based on single-arm studies without appropriate controls. The inclusion criteria for peer-reviewed publications show highly selected patients with good performance status and documented slow pace of tumor progression. Critics of ablative therapies have suggested that the long-term survival achieved by treatment of oligometastases is more a function of patient selection with slow tumor growth rather than the result of treatment intervention.
Randomized trials are, therefore, necessary to establish the usefulness of ablative treatment of oligometastatic disease, but such randomized trials are rare. One such completed randomized trial, Radiation Therapy and Oncology Group Trial 9508, compared whole brain radiotherapy (WBRT) with WBRT plus stereotactic treatment for patients with 1 to 3 brain metastases, and found an overall survival advantage only in patients with a single metastasis and those patients in the most favorable baseline recursive partitioning analysis prognostic group. Patients with inferior baseline prognostic factors did not achieve a survival benefit from stereotactic boost treatment.
It is unclear whether all patients with oligometastatic disease can benefit significantly from SABR, in terms of improved local control, improved survival, or improved quality of life. Although SABR generally results in successful ablation of each metastatic target, patients remain at high risk of further metastatic progression. Results from SABR for treatment of oligometastases in published studies appears promising, but these favorable results may be owing to patient selection, rather than treatment intervention, and are based on comparisons with historical controls. The benefit of comprehensive treatment of oligometastases can only be demonstrated conclusively in the context of a randomized trial.
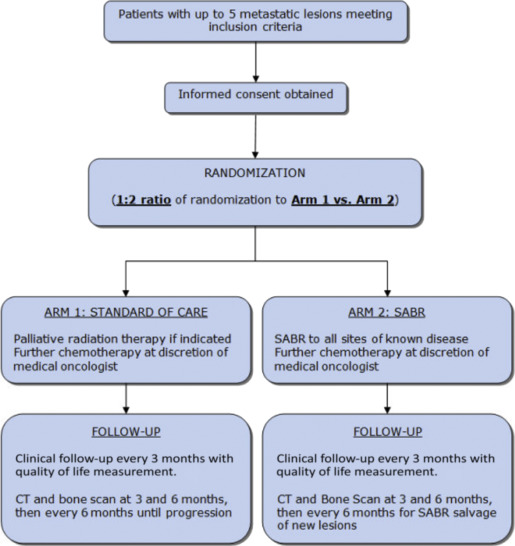
Several ongoing studies are attempting to address these concerns, including the multicenter, Stereotactic Ablative Radiotherapy for Comprehensive Treatment of Oligometastatic Tumors (SABR-COMET) international study, which aims to accrue 99 patients and provide preliminary evidence to assess the impact of a comprehensive oligometastatic SABR treatment program on overall survival and quality of life. After stratification by the number of metastases (1–3 vs 4–5), patients will be randomized between arm 1 (current standard of care treatment) and arm 2 (standard of care treatment plus SABR to all sites of known disease; Fig. 1 ). For patients receiving SABR, radiotherapy dose and fractionation including motion management assessment is defined rigidly and based on location of metastasis and adjacent critical structure tolerances. The primary endpoint is overall survival, and secondary endpoints include quality of life, toxicity, progression-free survival, lesion control rate, and number of cycles of further chemotherapy/systemic therapy.
This article touches on the clinical considerations and applications for the use of SABR with specific consideration for patients with oligometastatic non–small cell lung cancer (NSCLC).
Introduction
From the very onset of an oncologist’s clinical and specialty training, the concept of routine adherence to evidence-based medicine and clinical practice guidelines molds the physician to prescribe and administer therapy based on primary cancer site and stage. The oncologist follows established national and institutional pathways while simultaneously synthesizing the balance of documented disease presentation, patient age, performance status, and medical comorbidities to create a plan of care. Treatment is dynamically modified to disease response or progression, provided the patient remains an appropriate candidate for additional management. Successful eradication of disease earns professional and personal satisfaction, whereas progression of disease inherently necessitates the reevaluation of the therapeutic approach.
The rapid evolution of nontraditional treatments in the field of oncology medicine has afforded new opportunities for consideration of the inclusion of therapeutic approaches for isolated cancer lesions that extend beyond accepted guidelines and pathways. Minimally invasive surgical approaches, whether robot assisted or laparoscopic, often reduce the expected risks and complications previously considered barriers to their inclusion as part of cancer treatment. The evolution in clinical experience regarding the technical capabilities, applications, and toxicities for ablative radiotherapy to address specific sites of active disease remains an active area of clinical research and investigation. Radiofrequency or cryotherapy ablations offer additional opportunities for focused tissue destruction to address isolated foci of malignancy.
The oligometastatic disease state was first defined in 1995 and refers to a stage of disease, where cancer has spread beyond the site of origin, but is not yet widely metastatic. In such a state of limited metastatic disease burden, it is hypothesized that eradication of all sites of metastatic disease could result in long-term survival, or even cure, in a subgroup of patients. Ablation of metastatic deposits can be achieved surgically, or through stereotactic ablative radiotherapy (SABR), a new radiotherapy technology that delivers very large, hypofractionated doses of radiotherapy with high precision to small tumor targets, with high rates of local control.
Clinical evidence to support the presence of an oligometastatic state is emerging in both the surgical and SABR literature, for several tumor types. In a study of more than 5200 patients with lung metastases who underwent surgical resection, a 5-year survival of 36% was reported in patients who achieved a complete resection, which is much greater than would be expected for many patients with disseminated stage IV disease. Similarly, in patients treated with SABR for 1 to 3 lung metastases from a variety of primary tumors types, local control with SABR was 96% at 2 years, and the 2-year survival was 39%. The potential for long-term survival has been demonstrated in patients treated for oligometastases using surgery or SABR for metastatic lesions located in the liver, brain, bone, and adrenal glands. However, the risk of new metastatic disease, in either a new site or increase in clinical volume, remains high after ablative treatment, with reported rates up to 60% to 80%. Although SABR may be used as a further salvage treatment, often patient performance status and socioeconomic factors create functional limitations in the routine application for cancer treatment.
Despite the achievement of long-term survivors beyond statistical mean survivals with ablative treatment for oligometastatic disease, the level of evidence to support such treatments in routine clinical practice is weak in many cases, often based on single-arm studies without appropriate controls. The inclusion criteria for peer-reviewed publications show highly selected patients with good performance status and documented slow pace of tumor progression. Critics of ablative therapies have suggested that the long-term survival achieved by treatment of oligometastases is more a function of patient selection with slow tumor growth rather than the result of treatment intervention.
Randomized trials are, therefore, necessary to establish the usefulness of ablative treatment of oligometastatic disease, but such randomized trials are rare. One such completed randomized trial, Radiation Therapy and Oncology Group Trial 9508, compared whole brain radiotherapy (WBRT) with WBRT plus stereotactic treatment for patients with 1 to 3 brain metastases, and found an overall survival advantage only in patients with a single metastasis and those patients in the most favorable baseline recursive partitioning analysis prognostic group. Patients with inferior baseline prognostic factors did not achieve a survival benefit from stereotactic boost treatment.
It is unclear whether all patients with oligometastatic disease can benefit significantly from SABR, in terms of improved local control, improved survival, or improved quality of life. Although SABR generally results in successful ablation of each metastatic target, patients remain at high risk of further metastatic progression. Results from SABR for treatment of oligometastases in published studies appears promising, but these favorable results may be owing to patient selection, rather than treatment intervention, and are based on comparisons with historical controls. The benefit of comprehensive treatment of oligometastases can only be demonstrated conclusively in the context of a randomized trial.
Several ongoing studies are attempting to address these concerns, including the multicenter, Stereotactic Ablative Radiotherapy for Comprehensive Treatment of Oligometastatic Tumors (SABR-COMET) international study, which aims to accrue 99 patients and provide preliminary evidence to assess the impact of a comprehensive oligometastatic SABR treatment program on overall survival and quality of life. After stratification by the number of metastases (1–3 vs 4–5), patients will be randomized between arm 1 (current standard of care treatment) and arm 2 (standard of care treatment plus SABR to all sites of known disease; Fig. 1 ). For patients receiving SABR, radiotherapy dose and fractionation including motion management assessment is defined rigidly and based on location of metastasis and adjacent critical structure tolerances. The primary endpoint is overall survival, and secondary endpoints include quality of life, toxicity, progression-free survival, lesion control rate, and number of cycles of further chemotherapy/systemic therapy.