Study, year (Ref.)
N
No. cancers (%)
Mean follow-up (years)
Ipsilateral cancer (%)
Contralateral cancer (%)
Lobular histology (%)
Hutter, 1969 [12]
40
15 (33)
4–27
59
41
NR
Haagensen, 1971 [16]
22
9 (40)
9.5
55
44
NR
Wheeler, 1974 [17]
25
4 (16)
17.5
25
75
25
Andersen, 1977 [2]
44
13 (27.7)
15.9
50
50
NR
Haagensen, 1978 [1]
211
35 (16.5)
14
49
51
54
Rosen, 1978 [14]
84
29 (34)
24
60
50
33
Page, 1985 [4]
126
16 (12.7)
17.5
69
31
19
Page, 2003 [18]
161
25 (16)
16
68
20
NR
Chuba, 2005 [13]
4,853
350 (7.2)
10
46
54
23
Li, 2006 [15]
4,490
282 (6.2)
5.5
58
42
49
Hwang, 2008 [19]
148
4 (2.7)
4.1
75
25
0
Epidemiology
Since there are no specific physical or imaging findings for LN, it is most commonly noted as an occult lesion in surgical specimens or breast biopsies performed for other indications. This makes clarifying the actual overall incidence somewhat problematic. The prevalence of LN, in an otherwise benign breast biopsy, is approximately 0.02–3.8 % [1, 20–23]. Due to the fact that LN is an incidental finding, the actual prevalence of the disease may be much higher.
LCIS is most commonly diagnosed in women in the fifth decade of life, a decade earlier than those diagnosed with DCIS [1, 18]. The majority of women are premenopausal. Based on SEER data, the age-adjusted incidence of LN increased fourfold from 1978 to 1998 (from 0.9 to 3.2/100,000 person-yrs) [24]. Women between the ages of 50 and 59 experienced the greatest absolute increase in incidence over the study period. This increase is likely due to the higher use of screening mammography and increased frequency of breast biopsies for mammographic abnormalities [5, 24].
Histology
Lobular neoplasia is used to describe a spectrum of benign proliferation which encompasses LCIS and ALH. Histologically, LCIS is typically characterized by the proliferation of monotonous, discohesive cells, which fill the acini and cause significant distension [25, 26]. Pagetoid spread, in which the neoplastic cells extend along adjacent ducts and in between intact overlying epithelium and underlying basement membrane, may also be present [8, 27]. In ALH, the cells are cytologically similar to LCIS but with minimal distention of the acini (Fig. 10.1).
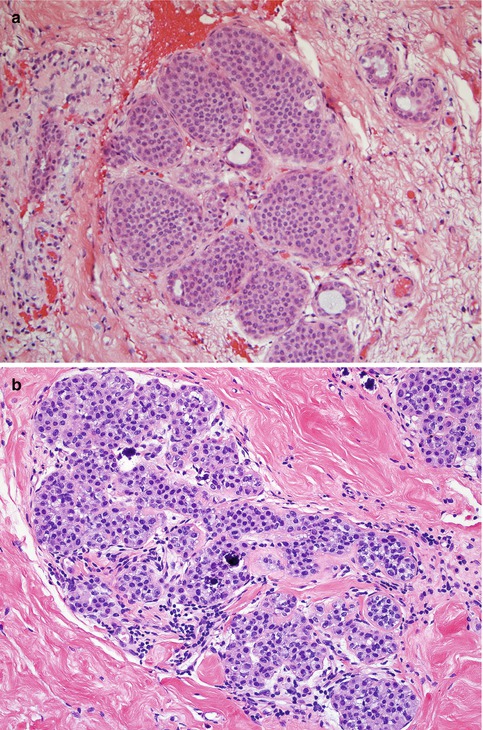

Fig. 10.1
Histopathologic appearance of lobular neoplasia (Reprinted with permission [28]). (a) Lobular carcinoma in situ. Note that the acini within the lobular unit are filled and show significant distension by the neoplastic proliferation. (b) Atypical lobular hyperplasia. Cytologically similar to LCIS, with dyshesive and uniform cells, but minimal distension of the acini
The above is a description of classic LCIS and is also referred to as type A cells. Type B cells are a well-recognized subtype of LCIS, with mild to moderately larger nuclei showing some increase in pleomorphism [27]. The entity described as pleomorphic LCIS (PLCIS) has cells with more marked pleomorphism and distinctly larger, eccentrically placed nuclei with nucleoli and eosinophilic cytoplasm. The cells of PLCIS often have central necrosis and calcification within granules [27, 29]. These lesions are often difficult to differentiate from DCIS, with staining for E-cadherin often helpful in differentiating PLCIS from DCIS.
E-cadherin is a transmembrane glycoprotein responsible for calcium-dependent cell-to-cell adhesion [30]. This protein is usually absent in lobular neoplasms such as LCIS but present in disease of ductal origin, such as DCIS. Lack of E-cadherin staining supports a diagnosis of LN. Lobular neoplasia consistently demonstrates estrogen and progesterone receptor positivity. However, Her2/neu receptor expression is variable and may be overexpressed in the PLCIS variant [8].
Although criteria exist for differentiating ALH from LCIS, the distinction can be fairly subjective, with significant intraobserver variability among pathologists when distinguishing between these entities. Rosai et al. [31] reported on a series of 17 cases that were evaluated by 5 expert breast pathologists. These lesions included ten ductal and seven lobular lesions, and the pathologists were asked to diagnose these lesions as typical hyperplasia, atypical hyperplasia, or carcinoma in situ. The 5 pathologists were not in unanimous agreement on a diagnosis for any of the 17 lesions. There were only three lesions in which four of the five pathologists agreed upon the diagnosis.
It has been suggested that lobular neoplasia should possibly be renamed “lobular intraepithelial neoplasia (LIN)” and further subdivided into three grades based upon morphology and an increased risk for the development of invasive cancer (LIN 1, 2, and 3). PLCIS would be described as LIN3 [32]. This modified and new classification system has yet to gain traction, possibly due to the risk of further confusing both clinicians and patients with LN. Also labelling LCIS a “cancer” may have unintended consequences, such as overtreatment. Moving forward, we should consider reserving the word cancer for “lesions with a reasonable likelihood of lethal progression if left untreated” as spelled out in a recent JAMA editorial [33].
Lobular Neoplasia Found at Core-Needle Biopsy
The current version of the NCCN treatment guidelines clearly outlines a treatment algorithm for the management of LCIS [34]. The recommendations state that when LCIS is found within the surgical specimen, no additional surgical intervention is necessary. However, when LCIS is encountered on needle biopsy, surgical excision should be performed to rule out a coexisting DCIS or possibly an invasive cancer. Many reports have been published to determine the actual rate of lesion upstaging with both LCIS and ALH, varying widely from 0 to 60 % [19, 28, 35–43] (Table 10.2).
Table 10.2
Upstage rate of lobular neoplasia on core-needle biopsy
ALH | LCIS | |||
|---|---|---|---|---|
Malignancies found on surgical excision | % Upgrade | Malignancies found on surgical excision | % Upgrade | |
Yeh, 2003 [35] | 1/12 | 8 | 0/3 | 0 |
Foster, 2004 [36] | 2/14 | 14 | 4/12 | 33 |
Elsheikh, 2005 [37] | 5/20 | 25 | 4/13 | 31 |
Margenthaler, 2006 [38] | 3/19 | 16 | 4/16 | 20 |
Brem, 2008 [39] | 21/97 | 22 | 17/67 | 25 |
Cangiarella, 2008 [40] | 1/18 | 6 | 2/20 | 10 |
Londero, 2008 [41] | 1/8 | 12 | 12/20 | 60 |
Hwang, 2008 [19] | 1/48 | 2 | 9/39 | 23 |
Rendi, 2012 [42] | 2/48 | 4.1 | 1/20 | 5 |
Shah-Khan, 2012 [28] | 1/81 | 1.5 | 1/20 | 5 |
Murray, 2013 [43] | 2/29 | 6.8 | 0/42 | 0 |
There are several possible reasons for such a wide variation in the percentage, such as the majority of the studies being retrospective in nature, often with a small number of patients. One limitation of many of these institutional reviews is the basic methodology, specifically focusing only upon the group that underwent surgical excision. By conducting a retrospective review of all cases that had LN on a preoperative needle biopsy that underwent surgical excision, the entire group of LN at the same institution that did not undergo an operation is not included in the analysis. This, of course, will affect the overall incidence of lesion upstaging. This will translate into an inherent selection bias toward the lesions that are excised versus those observed, with subsequent upstaging rates that are falsely elevated.
The other major design flaw of many reviews is that they do not account for radiographic and pathologic concordance, without eliminating those core biopsies that contain other high-risk lesions. Many core-needle biopsy specimens contain heterogenous samples, including other high-risk lesions such as atypical ductal hyperplasia, which is known to have a much higher upstage rate.
In the series by Londero et al. [41], complete excision of LN found on core-needle biopsy was advised, yet only one case of ALH was upstaged at surgical excision. This single mass lesion contained calcifications and the core-needle biopsy revealed ALH. Of the 12 upstaged cases of LCIS, 2 were pleomorphic calcifications extending over a 2-cm area and 6 were hypoechoic nodules [41]. In a recent prospective study of 85 consecutive core-needle biopsies for LN, 80 underwent excisional biopsy and carcinoma was identified in 3 % of concordant cases versus 38 % of discordant cases [43]. The importance of radiographic and pathologic concordance in allowing for an individualized approach to care of this disease cannot be overstated.
When considering whether to excise LN that is encountered on the original core-needle biopsy, it is reasonable to evaluate these situations on a case-by-case basis. The following situations are commonly encountered:
Scenario #1: ALH with No Other High-Risk Lesion, with Radiologic and Pathologic Concordance
Case 1
Forty-five-year-old female with a screening mammogram showing an area of suspicious microcalcifications measuring 5 mm in diameter reported as BIRADS category 4 – a suspicious abnormality. Stereotactic core-needle biopsy with an 11-gauge needle and a total of ten core samples reveal ALH. On further analysis of the final pathology, there are fibrocystic changes only, with some associated calcium in the benign fibrocystic ducts. There is a single focus of ALH. A clip is in good position and it appears on post-biopsy mammogram that all of the microcalcifications were removed.
Our recommendation for this patient would be for continued observation and a discussion of chemopreventive strategies, such as tamoxifen.
Concordance is achieved when the pathologic findings provide a sufficient explanation for the radiologic appearance. These lesions can generally be observed. It is recommended that the issue of concordance be carefully reviewed and that the surgeon, radiologist, and pathologist are in agreement with these findings. When evaluating the literature carefully, lesions of pure ALH that have demonstrated documented radiologic–pathologic concordance consistently have a low rate of upstaging to a malignancy (Table 10.3).
Table 10.3
Pure ALH with concordance and no other high-risk lesions
Study | Number of cases ALH | Total upstage | Upstage concordant only | Comments |
|---|---|---|---|---|
Hwang [19] | 48 | 1/48 (2 %) | 1 mass lesion upstaged to DCIS | |
Shah-Khan [28] | 81 | 1/81 (1.5 %) | 0/73 (0 %) | 1 discordant case upstaged to DCIS |
Rendi [42] | 48 | 2/48 (4.1 %) | 1/48 (2.1 %) | 1 of 2 lesions upstaged was a mass lesion noted to be discordant |
Murray [43] | 72 | 5/80 (6 %) | 2/72 (3 %) | 1 upgrade was a 2-mm DCIS, 1 was a 2-mm IDC |
3/8 discordant lesions (38 %) upstaged | ||||
Nagi [44] | 35 | 0/35 (0 %) | 0 % |
The data we have collected from the Mayo Clinic addresses the upstage rate of pure LN [28]. We performed a retrospective review evaluating all patients that underwent core-needle biopsy between 1993 and 2010. We excluded any core biopsy that contained an associated high-risk lesion such as PLCIS, ADH, radial scar, papilloma, flat epithelial atypia (FEA), ipsilateral invasive carcinoma, or DCIS. The final analysis included 184 cases, with 74 % of the core biopsies performed for suspicious microcalcifications, 21 % for a solid mass or nodule, and 5 % for an area deemed suspicious by breast MRI. We identified 147 (80 %) patients with ALH and 37 (20 %) with LCIS. Of these, 81 (55 %) of the ALH cases and 20 (54 %) of LCIS cases underwent definitive operative removal of the lesions.
The rate of upstage upon surgical excision for ALH was 1.5 % (1/81) and 5 % (1/20) for LCIS. The single ALH case that was upstaged had a discordant biopsy result and was upgraded to DCIS. Of the ALH lesions that had no other associated high-risk features and pathologic–radiographic concordance on needle biopsy, none were upstaged at the time of excisional biopsy (Table 10.4). These upstage rates are very low and consistent with other studies evaluating the upstage rates of pure LN that have undergone meticulous review of the pathologic–radiographic concordance [19, 42–44]. Similar findings were recently published from a prospective series by Murray et al. [43].
Table 10.4
Mayo Clinic data
ALH (%) | LCIS (%) | p-value | |
|---|---|---|---|
Upstaged at excision | 1/81 (1.5) | 1/20 (0) | 0.36 |
Concordant cases only | 0/73 (0) | 1/18 (5.6) | 0.2 |
Developed ipsilateral cancer | 2/112 (1.8) | 3/26 (11.5) | 0.04 |
Developed contralateral cancer | 3/112 (2.7) | 1/26 (3.8) | 0.57 |
Our series is somewhat unique in that it included the entire cohort of LN lesions, including those that were observed and did not undergo excisional biopsy. Follow-up data was available for 65 cases that were merely observed without surgical excision, with a mean follow-up period of 53 months. In patients with ALH, 1/51 (2 %) developed an ipsilateral cancer and for those with LCIS, 3/14 (21.4 %) developed an ipsilateral cancer. This ipsilateral breast event rate is high; however, it is worth noting that all patients with LCIS that developed a subsequent ipsilateral malignancy did so in a different quadrant than the initial biopsy, suggesting excisional surgical biopsy would not have influenced the future events. This is also a relatively small cohort, as the majority of patients with LCIS in our series did go onto excisional biopsy, thus caution is advised in making conclusions based on this subset of 14 patients. With regard to the contralateral breast, 2.7 % of patients with ALH and 3.8 % with LCIS developed a contralateral malignancy during the follow-up period.
All of the subsequent cancers that developed were invasive ductal carcinoma in origin. Although these rates appear to be much lower than the expected rate of development of malignancy in such patients with LN, the lifetime incidence for the development of ipsilateral or contralateral or malignancy was 21 % (31/145) for those with ALH and 29 % (10/35) for those with LCIS. These lesions are associated with an increased risk of future breast cancer and these patients should be under surveillance and strongly consider chemoprevention. This is discussed in more detail below.
Hwang et al. reported on a series of 48 patients with pure ALH, of which the upstage rate was 2 % [19]. Of note, the upgraded lesion was a biopsy performed for a mass lesion. This report also provided follow-up data for patients that did not undergo excision following a diagnosis of LN. The mean follow-up period was 49 months for 148 cases of LN without subsequent excision and demonstrated a 2 % incidence of subsequent ipsilateral carcinomas, including one case within a different site than the original biopsy identifying LN.
Brem et al. reported on a multi-institution series of LN [39], analyzing 67 LCIS and 97 ALH cases that underwent complete excision. The upgrade rate reported was substantial, 25 % for those with LCIS and 22 % (21/97) for those with ALH. However, of the 164 lesions excised, 74 (45 %) were noted to have radiologic–pathologic discordance. A major limitation of this study lies in the fact that there were several institutions included and no retrospective pathology overview, resulting in potential variability in the interpretation of LN. Rendi et al. reported on 68 patients with pure LN [42]. Two cases (4.1 %) of ALH were upstaged, with one being a mass lesion that was noted to be discordant. Thus, the true upstage rate of pure, concordant ALH in this series is likely closer to 2 %.
Extent of disease is also an important factor to be evaluated. Esserman et al. demonstrated that the extent of lobular neoplasia in a core biopsy specimen may predict whether or not excision is required [45]. They distinguished diffuse lobular neoplasia (>1 lobule per core affected) from focal lobular neoplasia (less than or equal to 1 lobule per core) and found that upgrades to malignancy were more likely to be associated with diffuse LN. Although the number of patients included was small (35 patients), these findings would support that in patients with simply a focus of LN, observation can be considered.
Scenario #2: ALH on Core-Needle Biopsy in the Presence of a Higher-Risk Lesion
Case 2
A 52-year-old female undergoes a screening mammogram that reveals a 1.5-cm diameter area of suspicious microcalcifications (BIRADS 4). A stereotactic biopsy with a 14-gauge core needle and four core samples reveals ALH and ADH with the microcalcifications associated with the atypical ducts. Post-biopsy mammogram reveals the clip in good position with many microcalcifications remaining.
Our recommendation would be for an excisional biopsy to obtain an adequate sample for final pathologic analysis to rule out an underlying occult malignancy. This is a conservative surgical excision. In general, this is a diagnostic procedure, and we are attempting to obtain an adequate representative sample to assure there is no upstaging. The rationale for excision in this case is twofold; the calcifications in question have not been adequately sampled, and a definitive diagnosis must be obtained due to the presence of a higher-risk lesion. As covered in another chapter, excisional biopsy is standard for ADH and results in approximately a 10–20 % upstage [46]. At the time of this publication, core-needle biopsy alone for ADH has not been able to stratify a low enough risk group that an occult cancer can be excluded. The management of such cases should be directed toward removal of the highest-risk lesion. The higher-risk lesion would trump the ALH and standard of care for the ADH would be followed.
The disadvantage of a more aggressive wire localized lumpectomy stems from the fact that lobular neoplasia is a risk factor for the development of breast cancer. This risk occurs in all quadrants and is bilateral; thus a more aggressive surgery, short of a bilateral mastectomy, does not prevent the potential development of a future malignancy. We are not prepared at the time of excisional biopsy to proceed to definitive mastectomy, reconstruction, or axillary staging if the lesion is upstaged. In the rare case where there is extensive calcifications throughout a large area or scattered throughout the breast and follow-up imaging may be inadequate, deciding what is an adequate sampling and observing can be unsettling and mastectomy may be an option. This should be the exception, not the rule.
The Role for Chemoprevention
Excisional biopsy reveals post-biopsy changes with a residual focus of ADH. The specimen radiograph revealed the clip and the residual calcifications.
In addition to future surveillance, it is recommended that patients with findings of atypia be counseled regarding their appropriateness for chemoprevention.
Prospective, randomized trials have been conducted to evaluate the effect of chemoprevention in reducing the development of breast cancer in patients at high-risk, including those with LCIS. The NSABP-01, known as the breast cancer prevention trial (BCPT), randomized 13,388 high-risk women to the selective estrogen receptor modulator (SERM), tamoxifen, versus placebo [47, 48]. Patients were determined to be at high risk if they were older than 60, had LCIS or atypical hyperplasia, or had a 5-year risk of developing breast cancer calculated as >1.66 % utilizing the Gail risk model.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree