Fig. 11.1
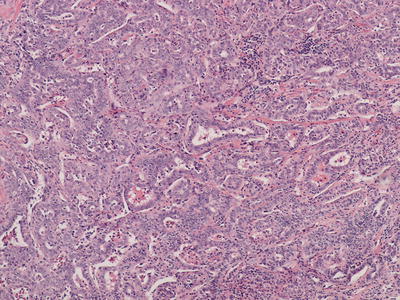
Hepatocellular carcinoma with eosinophilic cytoplasm and thick trabeculae outlined by endothelial cells
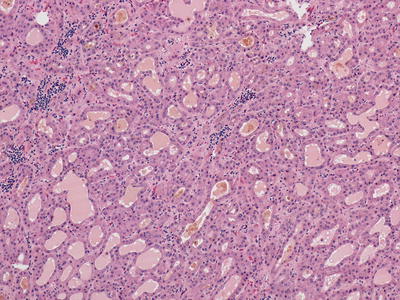
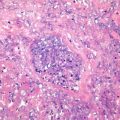
Fig. 11.2
Hepatocellular carcinoma with bile in the pseudoglands
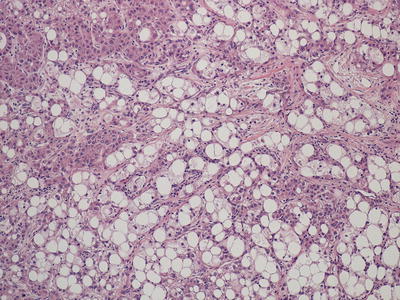
Fig. 11.3
Hepatocellular carcinoma with steatotic and ballooned tumor cells
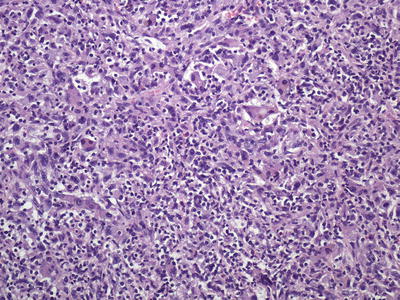
Fig. 11.4
Hepatocellular carcinoma , poorly differentiated without the characteristic eosinophilic cytoplasm or trabecular formation
11.1.1 Benign Hepatocellular Lesions
High-grade dysplastic nodules are considered premalignant lesions that arise in the cirrhotic liver. They are characterized by architectural atypia, i.e., nodule within nodule, and/or cellular atypia, the most frequent being an increase in cell density with increased nuclear to cytoplasmic ratio, so-called small cell change. These lesions may have thickened trabeculae, but not greater than three hepatocytes thick. The hepatocyte nuclei are round and isochromatic. Occasional unpaired arteries can be seen. It is important to remember that malignant transformation usually arise from the center of a high-grade dysplastic nodule and may not be represented when biopsy specimen is taken from the periphery of a lesion (Fig. 11.5). In the setting of genetic hemochromatosis, identification of nodules with decreased iron content compared to the surrounding liver (iron-free foci) indicates a proliferative, likely preneoplastic lesion [4].
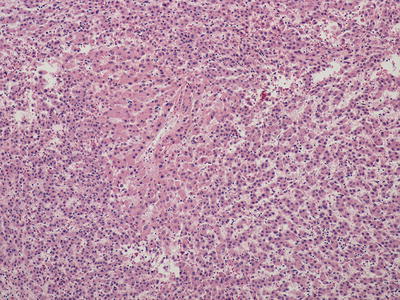

Fig. 11.5
Hepatocellular carcinoma arising in high-grade dysplastic nodule . A non-tumorous area still remains in the center
Hepatocellular adenomas (HCAs) are benign clonal proliferations of hepatocytes that are encountered in non-cirrhotic livers. Certain subtypes of HCAs have the potential to undergo malignant transformation, which will be described in more detail below. Focal nodular hyperplasia is a benign non-clonal proliferation of hepatocytes secondary to vascular changes and these lesions are not associated with significant malignant potential.
Currently, HCAs are classified into four distinct subtypes with differing clinical, morphological, immunohistochemical, and molecular features. These subtypes are designated as hepatocyte nuclear factor 1 alpha-mutated (H-HCA ), inflammatory (I-HCA ), and beta-catenin-mutated (b-HCA and b-IHCA) [5]. Hepatocellular adenomas that do not fit within these three groups are designated unclassified (U-HCA ). H-HCAs usually arise sporadically or in the setting of maturity-onset diabetes of the young and are characterized histologically by steatosis and show no significant cytologic atypia. These tumors have no significant potential for malignant transformation. By immunohistochemistry, these tumors demonstrate loss of staining with liver fatty acid-binding protein (Fig. 11.6a, b), which helps in separating H-HCA from steatotic vaguely nodular HCC . b-HCAs are hepatocellular adenomas with malignant potential that show nuclear staining for beta-catenin and/or diffuse cytoplasmic staining with glutamine synthetase. These adenomas occur more frequently in men and often show cytologic atypia (Fig. 11.7a, b). I-HCA tumors may be associated with systemic inflammatory disorders and may act as a source of systemic inflammation. These tumors are characterized by a chronic inflammatory infiltrate, dilated and/or telangiectatic sinusoids, and portal tract-like structures which demonstrate ductular reaction but do not contain native bile ducts (Fig. 11.8a). These tumors show positive staining for the inflammatory markers, serum amyloid A protein and/or C-reactive protein (Fig. 11.8b). A subset of I-HCAs that harbor a beta-catenin mutation (b-IHCA) have a potential for malignant transformation. As the understanding of the malignant potential for subgroups of HCAs has grown, biopsy is becoming more important in the decision-making process of managing these patients. An immunohistochemical panel consisting of beta-catenin, glutamine synthetase, liver fatty acid-binding protein, serum amyloid A, and C-reactive protein is recommended to appropriately classify the tumor. If limited tissue is available, staining with glutamine synthetase is the most useful in determining malignant potential and has the added benefit of ruling out focal nodular hyperplasia , which has a characteristic “maplike” staining pattern (see below). In addition, glutamine synthetase immunoreaction of centrilobular hepatocytes is also useful in identifying non-lesional areas.
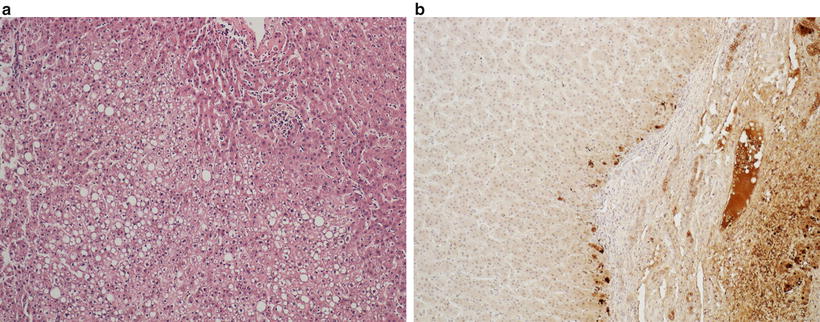
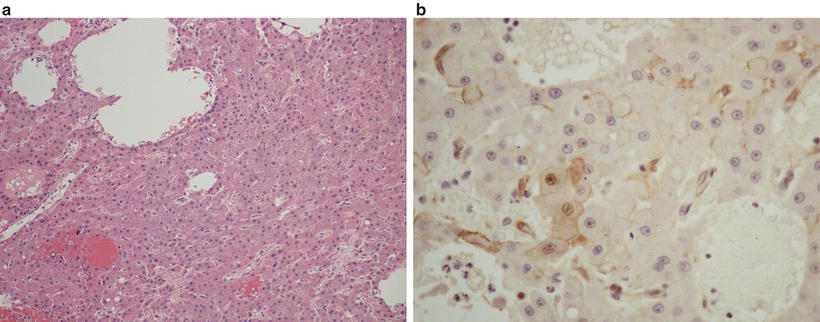
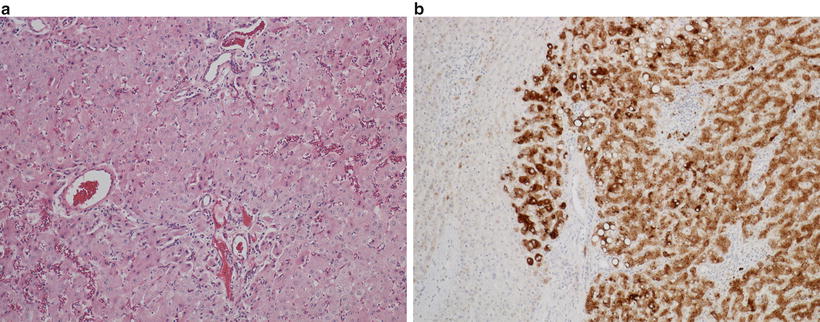

Fig. 11.6
Hepatocellular adenoma with steatosis, typically seen in HNF1α mutation (a); liver fatty acid-binding protein immunostain is absent in the adenoma (left) but is positive in the surrounding liver parenchyma (b)

Fig. 11.7
β-catenin-mutated hepatocellular adenoma with atypia (a) and nuclear immunostaining for β-catenin (b)

Fig. 11.8
Inflammatory hepatocellular adenoma with telangiectatic vessels and mild inflammation (a) and diffuse serum amyloid A immunostain of the adenoma (right) (b)
Focal nodular hyperplasia (FNH ) is a benign non-clonal hepatocellular proliferation that develops in response to vascular changes within the liver. These tumors usually arise in young women without underlying liver disease. FNH has a classic pattern on imaging, demonstrating central scar, and most cases do not require biopsy for diagnosis. Fibrolamellar carcinoma is a malignant hepatocellular neoplasm that also often arises in young women in the absence of known liver disease and also often shows a central scar on imaging studies. Liver biopsy may be indicated in differentiating these tumors and will be discussed below. The inflammatory subtype of HCA can also mimic FNH on imaging and histology. Because HCAs have the potential for malignant transformation, especially when they contain a beta-catenin mutation, and carry a high risk for bleeding, their differentiation from FNHs on needle biopsy is essential. On histology, FNH consists of a nodular proliferation of cytologically bland hepatocytes with arteries which demonstrate irregular and eccentric wall thickening. The larger vessels are embedded in fibrous septa, which do not contain bile ducts, though ductular reaction can be seen along their periphery (Fig. 11.9a). It is important to have the appropriate clinical information (age, sex, and presence or absence of underlying liver disease) when considering the diagnosis of FNH on needle biopsy. Depending on the portion of the lesion that is biopsied, the lesion can mimic cirrhotic liver or an HCA . Recognition of eccentrically thickened arteries is key to making the diagnosis; however, these are not always sampled. Immunohistochemical stain for glutamine synthetase can be very helpful, especially if these eccentrically thickened vessels are not seen on the biopsy. Glutamine synthetase demonstrates a “maplike” staining pattern of hepatocytes away from the vascularized fibrous septa with absence of staining of periseptal regions [6] (Fig. 11.9b). This can be used to distinguish these lesions from HCAs which will either be diffusely positive in b-HCAs, or spotty, or negative in other subtypes of HCAs . The I-HCA subtype will demonstrate diffuse staining for serum amyloid A protein and/or C-reactive protein, which is not seen in FNH .
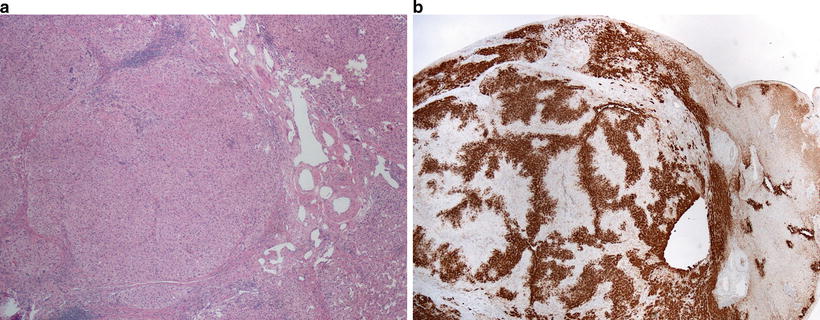

Fig. 11.9
Focal nodular hyperplasia with dystrophic blood vessels and fibrous septa (a) and characteristic maplike pattern of glutamine synthetase immunostain (left) (b)
11.1.2 Well-Differentiated Hepatocellular Carcinoma
Well-differentiated HCC is characterized by tumor cells with relatively abundant eosinophilic cytoplasm with central round nuclei and containing nucleoli. Well-differentiated HCC generally demonstrates trabecular or pseudoglandular growth patterns. The pseudoglandular patterns of HCC can be readily distinguished from the nonneoplastic liver by pattern alone, while the trabecular pattern can present more difficulty, especially on a small biopsy (Fig. 11.10a, b) [7]. In general, a lesion with trabeculae more than three hepatocytes thick indicates a malignant lesion. Additionally, identifying unpaired arteries (i.e., arteries without an accompanying bile duct) also suggests a neoplastic process [8], though this feature does not help in distinguishing HCA or high-grade dysplastic nodule from HCC . Stromal invasion is a histologic feature that, when present is indicative of malignancy in a vaguely nodular lesion. Stromal invasion is characterized by neoplastic hepatocytes invading a portal tract or fibrous septum (Fig. 11.11) [9]. Crystal blue or trichrome stains may be useful in identifying areas of stromal invasion [10]. Steatosis may be present in well-differentiated HCC and is observed in 40% of vaguely nodular HCC (Fig. 11.12). The International Consensus Group for Hepatocellular Neoplasia lists the following features that may be present in various combinations in HCC : increased cell density to two times the surrounding liver tissue with increased nuclear to cytoplasmic ratio and irregular thin trabeculae pattern, varying number of portal tracts within the tumor, pseudoglandular pattern, diffuse fatty change, and varying numbers of unpaired arteries. While these features can be helpful, many can also be seen in high-grade dysplastic nodules and individually are thus not specific for HCC . If present on the biopsy specimen, stromal invasion remains the most useful histologic feature of malignancy [11]. Several ancillary studies can be useful in supporting the diagnosis of HCC . Reticulin stain outlines the trabeculae of normal hepatocytes, while this staining pattern can be lost or altered in malignant processes (Fig. 11.10b) [12]. Reticulin stain can also be useful in assessing the thickness of the trabeculae. The hepatic sinusoids in the nonneoplastic liver are lined by a modified endothelium that does not stain for CD34, while the endothelial lining cells are often altered resulting in immunoreactivity for CD34 in HCC [8, 13]. A panel of three immunostains consisting of glypican-3, heat shock protein 70, and glutamine synthetase has been devised which demonstrates a 49% sensitivity and a 100% specificity in differentiating early HCC from high-grade dysplastic nodule on needle biopsy when any two markers are positive [14]. A panel of two immunostains of glypican-3 and heat shock protein 70 also showed a 67% sensitivity and 100% specificity in distinguishing HCC from HCA when either marker was positive. Glutamine synthase is not useful in this setting [15]. Of note, cholangiocarcinoma can also demonstrate trabecular architecture, especially at its periphery, though usually these tumors demonstrate fibrosis in the center of the tumor, and the tumor cells usually lack nucleoli (Fig. 11.13).
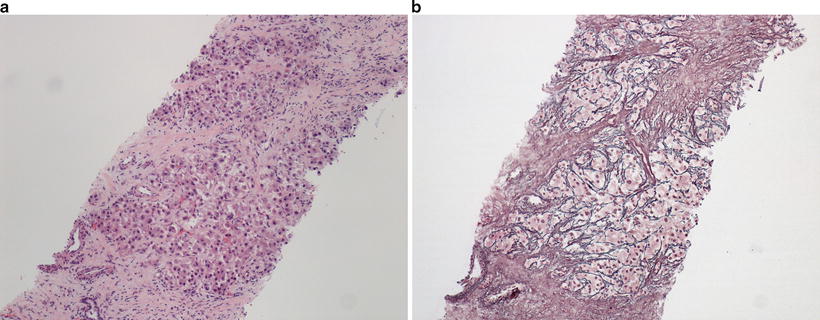
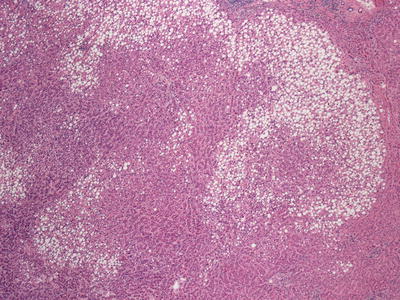
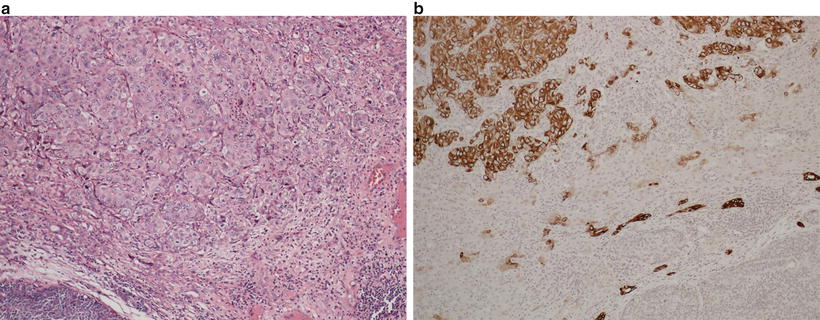

Fig. 11.10
Well-differentiated hepatocellular carcinoma (a) with altered but not decreased reticulin fibers (b)

Fig. 11.11
Well-differentiated hepatocellular carcinoma with stromal invasion of tumor cells in the portal tract

Fig. 11.12
Vaguely nodular hepatocellular carcinoma with steatosis and ill-defined borders

Fig. 11.13
Trabecular-like arrangement of tumor cells at the periphery of intrahepatic cholangiocarcinoma (a). Tumor cells are positive for CK7 (b)
Fibrolamellar carcinoma is a rare malignant liver tumor with characteristic clinical and morphologic features. These tumors usually arise in young patients with no known underlying liver disease. Recently, a DNAJB1-PRKACA chimeric transcript has been identified in fibrolamellar carcinoma which may play a role in the pathogenesis [16]. On imaging, these tumors often show a central scar, similar to FNH . On histology, these tumors are very distinctive. They are characterized by large oncocytic tumor cells with prominent nuclei within a dense lamellated collagenous background (Fig. 11.14). Some tumor cells may contain large amphophilic cytoplasmic inclusions termed “pale bodies.” [17] These tumors demonstrate hepatocellular differentiation, staining positively for HepPar-1. Unlike HCC , these tumors are almost universally positive for CK7 [18], EMA [19], and CD68 [20] (Fig. 11.15), and many also show positivity for other biliary markers such as B72.3, CK19, EpCAM, or monoclonal CEA by immunohistochemistry [19].
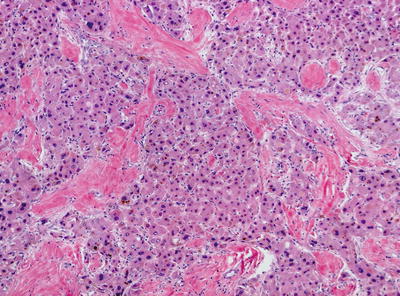
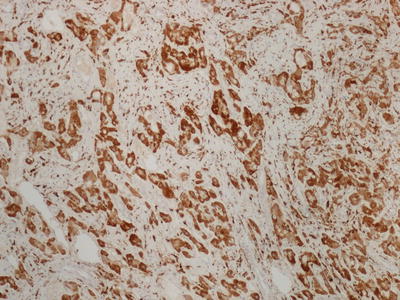

Fig. 11.14
Fibrolamellar carcinoma with large eosinophilic cells separated by hyaline fibrous septa

Fig. 11.15
Fibrolamellar carcinoma with CD68 positive tumor cells
Hepatoblastoma is the most common primary malignant liver tumor in infancy and young children. They rarely occur in older children or adults. Hepatoblastoma can be classified as epithelial type or mixed epithelial and mesenchymal type. The epithelial type can be further subdivided into pure fetal, mixed fetal and embryonal, mixed fetal and macrotrabecular, and mixed fetal and small cell undifferentiated, while the mixed epithelial and mesenchymal type can be further divided into those with and without teratoid features [21]. The fetal morphology consists of an alternating pattern of pale and darker staining epithelial cells resembling hepatocytes (Fig. 11.16). The embryonal type consists of angulated darker cells with high nucleus to cytoplasm ratio arranged in sheets or clusters or singly. The macrotrabecular subtype consists of thickened trabeculae (more than ten cells thick), and the small cell undifferentiated subtype consists of discohesive small round blue cells with minimal cytoplasm. The mixed epithelial and mesenchymal type consists of an epithelial component plus a mesenchymal component, such as mature or immature fibrous tissue, cartilage, or osteoid-like tissue. Mixed tumors with other mesenchymal and epithelial components such as skeletal muscle, bone, cartilage, and stratified squamous epithelium are considered teratoid. Differentiating fetal subtype of hepatoblastoma , especially when macrotrabecular component is present, from a well-differentiated HCC , can be difficult on a biopsy specimen, especially in older children or adolescents in which either type of tumor may be seen [22]. Identification of the pattern of alternating pale and darker staining cells is a key feature in diagnosing the fetal subtype of hepatoblastoma in these cases.
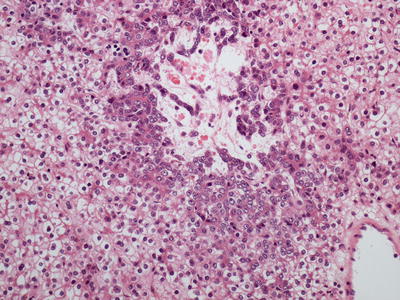

Fig. 11.16
Hepatoblastoma with characteristic darker and lighter-stained tumor cells
11.2 Biopsy Diagnosis of Cholangiocarcinoma, Combined Hepatocellular-Cholangiocarcinoma , Mixed Primary Liver Carcinoma, and Benign Biliary Proliferations
Intrahepatic cholangiocarcinoma (ICCA) usually arises in a non-cirrhotic liver and less commonly in hepatitis B, hepatitis C, and alcoholic-related cirrhosis. It can be subdivided into mass-forming, periductal infiltrating, and intraductal types. The periductal infiltrating type is characterized by tumor growth along the biliary tree, without a clear mass lesion, while the intraductal type is characterized by a papillary growth of biliary epithelium within the duct lumen which may cause cystic dilatation (Figs. 11.17 and 11.18). These types are much less common than the mass-forming types and may require resection in order to appreciate the architecture and establish the diagnosis. Mass-forming ICCA is often indistinguishable from metastatic carcinoma and less frequently from hepatocellular carcinoma on imaging, and a biopsy may be required for diagnosis.
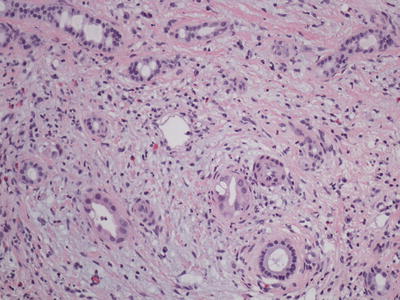
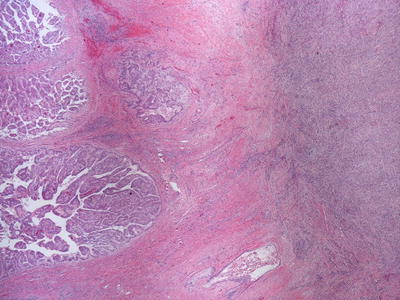

Fig. 11.17
Cholangiocarcinoma with periductal invasion

Fig. 11.18
Intraductal papillary cholangiocarcinoma
The diagnosis of ICCA is essentially a diagnosis of exclusion as metastatic lesions must be ruled out clinically and/or histologically. Several morphologic patterns can be seen in cholangiocarcinoma as these tumors can range from well to moderately to poorly differentiated carcinoma. The well-differentiated tumors are characterized by proliferation of regular glands with cuboidal or columnar lining and round regular nuclei. Moderately differentiated tumors may show more tortuous or less defined glands and are often lined by cells with more atypical nuclei (Fig. 11.19). Poorly differentiated tumors may show cells with scanty cytoplasm arranged in sheets, nests, trabeculae, or occasionally cribriform structures sometimes with comedonecrosis (Fig. 11.20). There is no obvious glandular formation. Undifferentiated cholangiocarcinomas consisting of markedly atypical cells with no discernable architectural structure are only rarely seen. Some tumors may show features more frequently seen in hilar and extrahepatic cholangiocarcinomas , including more pronounced mucin production, large glands with tall columnar cells, infiltration of the portal structures, and papillary growth pattern. These may represent tumors that originated as intraductal papillary neoplasms and progressed to form a mass lesion. ICCAs almost always initiate desmoplastic reaction. Interestingly, some ICCAs are comprised of polygonal eosinophilic tumor cells arranged in a prominent trabecular growth pattern, and these tumors may be difficult to distinguish from HCC . Trabecular growth may also be seen at the periphery of typical cholangiocarcinoma (Fig. 11.13). Helpful features in distinguishing these tumors from HCC include a more prominent fibrous stroma, especially in the center of the tumor, and lack of nucleoli. Bile production by the tumor is definitive evidence of hepatocellular, rather than biliary differentiation, although bile trapping sometimes is seen in ICCA. Immunohistochemical stains can usually resolve any difficult cases. Immunohistochemical evidence of hepatocellular carcinoma include positive staining for HepPar-1, cytoplasmic staining for TTF-1 (certain clone of antibody such as 8G7G3/1), and canalicular staining pattern with CD10 and pCEA. Cholangiocarcinomas are positive for CK7 and negative for the above markers of hepatocytic differentiation [7]. Of note, HCC can occasionally show focal positive staining for CK7, particularly the scirrhous type, a rare variant which is characterized by a dense fibrous stroma which may also demonstrate less staining with HepPar-1 [23].