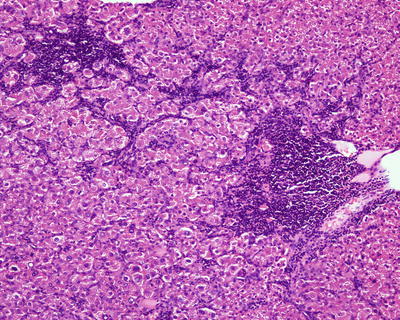
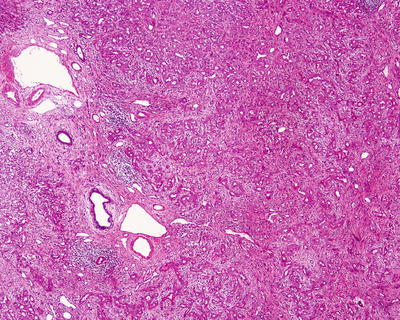
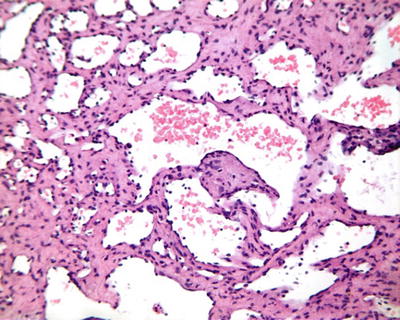
Fig. 6.1
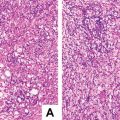
(a) The section of a huge mass is gray-red, soft, and homogeneous in texture. (b) A huge mass with a gray-green section. (c) HCA with hemorrhage. (d) Mutiple HCA of a case of glycogen storage; the section of surrounding liver tissue is yellow
6.1.1.4 Microscopic Features
HCA is a type of benign tumor due to proliferation of hepatocytes, and the tumor cells are similar to normal liver cells without obvious atypia that karyoplasmic ratio is not increased and nuclear division is rarely seen under the microscope. Fatty degeneration and hyaline degeneration can be found in the tumor cells, the cytoplasm of which contains lipofuscin and bile particle. The disorderly trabecular arrangement of the tumor cells is often 1–2 hepatocytes thick, with visible pseudoglandular structure occasionally and interstitial thin-walled veins; orphan arterioles have no concomitant bile ducts. The lesions are often accompanied by bleeding, necrosis, fibrosis, purpura, and other changes. The surrounding liver tissue is without cirrhosis, with fatty degeneration in some cases. Glycogen storage appears in a few cases with a pale hepatocellular cytoplasm, a clear membrane, and a lucent nuclear.
HCA can be divided into four different molecular types according to WHO classification, and each molecular type of HCA has corresponding clinical, imaging, pathological, and moleculobiological characteristics.
- 1.
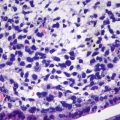
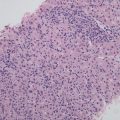
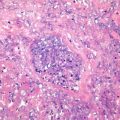
HNF1α mutation-inactivated HCA (H-HCA). H-HCA accounts for 35–40% of all the HCA cases. HNF1α (hepatocyte nuclear factor 1 α) gene is a tumor suppressor gene located on chromosome 12q24, encoding HNF1 protein, associated with the differentiation of the liver cells. H-HCA mutations include somatic type (90%) and germ line cell type (10%), both resulting in the production of a nonfunctional protein HNF11α which facilitates fatty degeneration and hepatocellular proliferation. Approximately 90% of the patients are young females with a history of oral contraceptives, while germ line cell type H-HCA are primarily seen in patients complicated by MODY-3 or young patients with familial adenomatous polyposis rather than a history of oral contraceptives. From the perspective of the characteristics of morphological pathology, H-HCA generally is a lobulated mass, with single or multiple lesions. Under the microscope, the boundary is clear and the arrangement of the liver plates is regular. The liver cells are enlarged with a small and anachromasis nucleus, obvious fatty degeneration, and no atypia or infiltration of inflammatory cells (Fig. 6.2). No dilation of the hepatic sinusoid. Some cases showed abundant lipofuscin (Fig. 6.3) with normal or fatty degenerated liver cells around the tumor. FABP is a gene positively regulated by HNF1A, encoding liver fatty acid-binding protein (L-FABP) positively expressed in normal liver tissue but markedly decreased in H-HCA. Immunohistochemical staining showed β-catenin stained on the cell membrane, almost complete negative or low expression of L-FABP, and intensive positive expression in the surrounding normal liver cells. L-FABP is a diagnostic marker of H-HCA with both 100% specificity and sensitivity. HCA does not express C-reactive protein (CRP) or serum amyloid A (SAA). GS stained the perivascular region, while focal GS positive expression can be found in the surrounding liver tissue. Focal expression of CD34 is also observed (Fig. 6.4) [11]. The risk of malignant transformation for H-HCA is lower than that of other types of HCA.
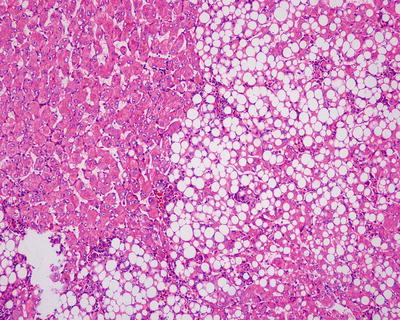
Fig. 6.2
HNF1α mutation-inactivated HCA (H-HCA). The boundary is clear, hepatic cells with obvious fatty degeneration and no atypia, without infiltration of inflammatory cells
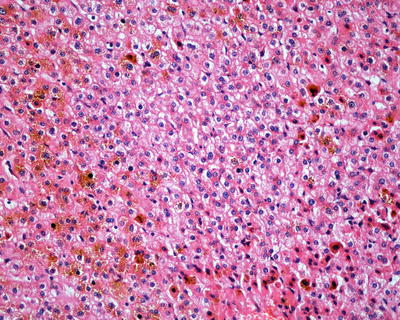
Fig. 6.3
HNF1α mutation-inactivated HCA (H-HCA) abundant lipofuscin in the tumor tissue
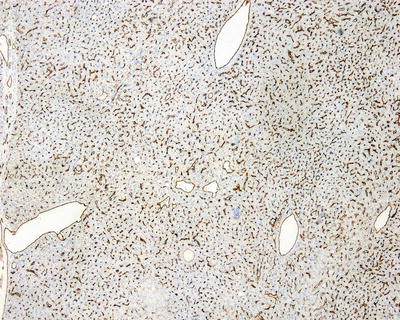
Fig. 6.4
Immunohistochemistry expression of CD34. Focal-patchy-diffused positive, negative in some area
- 2.
β-Catenin mutant-activated HCA (B-HCA). B-HCA often occurs in males, which is associated with glycogen storage disease and male hormone use. This type accounts for 10–15% of all the HCA cases with malignant potential. To date, the Wnt pathway associated with beta-catenin takes part in the genesis of adenomas. No HNF1α mutation has been found in B-HCA, but it can have both mutations of gp130 and GNAS, and about 50% of β-catenin mutant adenomas are inflammatory ones. B-HCA is often solitary nodules, while in cases with glycogen deposition disease, the multiple lesions are observed. Histologically, B-HCA often had no fatty degeneration or infiltration of inflammatory cells (Figs. 6.5 and 6.6), while the liver cells are atypia, enlarged cellular volumes, and irregular nuclei, and the liver plates thicken to form pseudoglandular structure, which sometimes makes it difficult to distinguish it from well-differentiated HCC . Immunohistochemical staining showed high expression of β-catenin and glutamine synthetase (GS) in B-HCA. β-Catenin was nucleus positive and often observed in only a small number of nuclei which needs careful investigation. GS was diffuse and homogeneous positive in the cytoplasm (Fig. 6.7), which is different from the map-like expression pattern of FNH . Though the combination of β-catenin and GS antibodies would increase the specificity for the diagnosis of B-HCA (100%), its sensitivity is relatively poor (75%–85%) [12]. Considering potential malignant transformation of B-HCA, molecular pathological screening of β-catenin mutation is necessary in HCA cases with no overexpression of β-catenin target genes or HNF1α mutation.
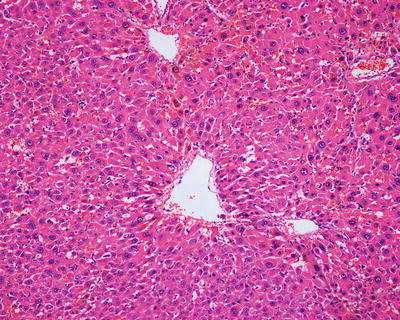
Fig. 6.5
β-Catenin mutant-activated HCA (B-HCA). B-HCA had no fatty degeneration or infiltration of inflammatory cells, while the liver cells are atypia
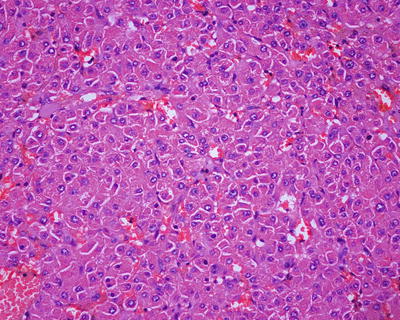
Fig. 6.6
β-Catenin mutant-activated HCA (B-HCA). B-HCA had no fatty degeneration or infiltration of inflammatory cells, while the liver cells are atypia

Fig. 6.7
β-Catenin mutant-activated HCA (B-HCA). GS was diffuse and homogeneous positive in the cytoplasm
- 3.
Inflammatory hepatocellular adenoma (I-HCA). I-HCA is the most common type of HCA, accounting for 40–55% of all HCA cases and also known as vascular expansion type of hepatic adenoma, which is closely related to obesity, alcohol consumption, and liver steatosis, and almost all I-HCA cases are found in young and middle-aged women with a history of oral contraceptives. The tumor is vulnerable to rupture and hemorrhage, generally with no nuclear atypia and low risk of malignant transformation, which can be divided into two subtypes.
- (1)
Inflammation with β-catenin activation: Sixty percent of the inflammation-type HCA cases contain gp130 (IL-6ST) somatic frameshift mutation, which is responsible for binding to IL-6 to activate the downstream inflammatory signal pathway of STAT3. And when gp130 mutation occurs, it will automatically activate the downstream inflammatory signaling pathways leading to inflammation without the ligand IL-6 [13]. β-Catenin staining is positive or GS staining shows diffusely positive.
- (2)
Inflammation without β-catenin activation: A small part (10%) of inflammation-type HCA may contain both β-catenin gene and gp130 mutations with mild malignant potential [14]. β-Catenin staining shows positive stained cell membrane and GS stains perivascular region.
- (1)
Fifty percent of the patients demonstrate elevated serum C-reactive protein and accelerated erythrocyte sedimentation rate, and fever and anemia can be observed in rare cases which can be eliminated after resection of the tumors. In general, I-HCA can be solitary or multiple with clear boundaries to the surrounding tissue. The tumor is soft and its cross section is heterogeneous, with no central scar. Histological characteristics of I-HCA include proliferation of liver cells, significant expansion or purpura of the liver blood sinus, focal or diffuse infiltration of inflammatory cells in the tumor (Figs. 6.8 and 6.9), visible fiber interval, dysplasia of the bile ducts, and solitary arteries (Fig. 6.10). In addition, various degrees of the fatty degeneration can be found in the liver cells. Immunohistochemistry showed overexpression of CRP (Fig. 6.11) or SAA. The proliferated bile ducts can sometimes be distinguished with expression of CK7 (Fig. 6.12) or CK19 immunohistochemical staining.
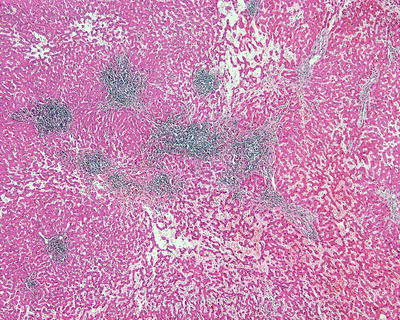
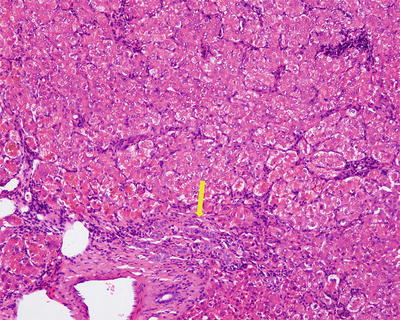
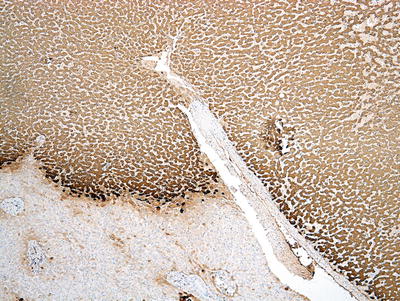

Fig. 6.8
Inflammatory hepatocellular adenoma (I-HCA) proliferation of liver cells, significant expansion of the liver blood sinus, and focal infiltration of inflammatory cells in the tumor

Fig. 6.10
Inflammatory hepatocellular adenoma (I-HCA). Visible fiber interval and dysplasia of the bile ducts in the tumor

Fig. 6.11
Inflammatory hepatocellular adenoma (I-HCA). Immunohistochemistry showed overexpression of CRP

Fig. 6.12
Inflammatory hepatocellular adenoma (I-HCA). CK7 showed the proliferated bile ducts
- 4.
Undifferentiated HCA. This type of HCA accounts for less than 10% of all the cases, without any mutations or histological or immunohistochemical characteristics involved in the previously discussed types, and no explicit predisposing factors or clinical features have been determined currently.
It is worth noting that molecular pathological changes of Chinese HCA patients can be different from that in the Western countries. In the Department of Pathology of East Hepatobiliary Surgery Hospital of Second Military Medical University, we studied three HCA marker genes including HNF1α, β-catenin, and gp130 in 36 cases of HCA [15], and the gene sequencing showed that all HCA contained HNF1α mutation with a significantly higher mutation rate by 35–40% than that reported in Europe. Furthermore, we also found several new hot spots of HNF1 gene mutation which were not included in the European reports. However, no mutations of β-catenin or gp130 genes in the HCA tissues have been found in our experience with negative β-catenin nuclear staining in immunohistochemical investigation. Varying incidences of inflammation type of HCA with β-catenin activation have been reported abroad, ranged 0–17%. In addition, the canceration rate of Chinese HCA cases is reported lower than that in Western countries [17]. Therefore, we should put more efforts into the researches on the tumor molecular pathology of Chinese HCA cases to determine molecular markers for molecular typing.
6.1.1.5 Differential Diagnosis
Differential diagnosis of HCA and focal nodular hyperplasia and fibrolamellar hepatocellular carcinoma is shown in Table 6.1.
Table 6.1
Comparison of focal nodular hyperplasia (FNH ), hepatocellular adenoma (HCA), and fibrolamellar hepatocellular carcinoma (FLC)
Items | FNH | HCA | Highly differentiated HCC |
|---|---|---|---|
Incidence | 0.6–3% [1] | Lower than the incidence of FNH by 3–10 times | 0.5–5.8% of the incidence of HCC |
Predilection age | 39 years on average | 38 years on average | 25 years on average |
Male/female ratio | 1.6∶1 | 2.3∶1 | ~ 1∶1 |
Clinical symptoms | Few | None or sudden abdominal pain | None or abdominal discomfort or jaundice |
Hepatitis | Few | Few | Few |
Serum AFP | Negative | Negative | Negative |
Mass number | Single or multiple (10%) | Single (70%) | Single |
Mass size | <5 cm in diameter | >5 cm in diameter | >10 cm in diameter |
Boundary | Clear | Clear | Clear |
Transection | 40.5% cases with stellate scar or nodular | Homogeneous, mostly without stellate scar | Nodular, 75% cases with stellate scar [18] |
Hemorrhage and necrosis | Rare | Tumor rupture and hemorrhage | Necrosis, calcification |
Hepatocells in the mass | No atypia | No or mild atypia | Obvious atypia, large cells with eosinophilic cytoplasm |
Vessel | Vascular malformation with thick vessel wall | Often thin-walled vessels | Thin-walled vessels |
Fiber bundles | Radial | Often none | Parallel or lamellar |
Bile ductule | Obvious hyperplasia | Often none, Observable in cases with vascular expansion | None |
Kupffer cell | Observable | Decrease | Disappear |
Surrounding liver tissue | No cirrhosis or fibrosis | No cirrhosis or fibrosis | No cirrhosis or fibrosis |
6.1.1.6 Treatment and Prognosis
The major complications of HCA are tumor rupture and malignant transformation. The occurrence of rupture and hemorrhage is directly related with tumor size. For adenomas larger than >5 cm in diameter, the risk of rupture and bleeding is higher. The risk of malignant transformation of hepatic adenomas is about 7%, predominantly found in males with β-catenin mutation. So far, no uniform standards for the treatment of hepatic adenoma have been determined. Nauh et al. [19] proposed the principles for the diagnosis and treatment of hepatic adenoma and suggested indications for surgery, including: ➀ adenoma with a maximum diameter > 5 cm, ➁ male, and ➂ female, adenoma with a maximum diameter <5 cm, with manifestation of B-HCA. Indications for follow-up by MRI include: female, MRI shows typical features of H-HCA or I-HCA and adenoma in small volumes. And if MRI shows no typical images of H-HCA or I-HCA, a biopsy should be conducted for screening of β-catenin mutation to evaluate the risk of malignant transformation.
6.1.2 Hepatic Adenomatosis
Wen-Ming Cong4 and Yu-Yao Zhu4
(4)
Department of Pathology, Eastern Hepatobiliary Surgery Hospital,, Second Military Medical University,, Shanghai,, China
6.1.2.1 Pathogenesis and Mechanism
Hepatic adenomatosis was first described by Flejou et al. [20] in 1985, and no consistent standard for the tumor number of hepatic adenomatosis has been determined. Some scholars suggested that hepatic adenomatosis can be diagnosed according to the number of more than ten and the involvement of left and ring lobes of the liver without any history of steroid medication or glycogen storage disease [21, 22]. However, we support the diagnostic criteria proposed by Wesley et al. [23] in 2008 including more than four tumor lesions because of the scarcity of more than four tumors in hepatic adenomatosis. Despite the low incidence, there have been 177 reported cases of hepatic adenomatosis [24]. Many studies suggested the major difference of hepatic adenomatosis from hepatocellular adenoma that the former is mainly found in young or middle-aged females with oral contraceptive medication.
6.1.2.2 Clinical Features
The disease is more common in young and middle-aged women with an average age of 32 years old. However, Babaoglu et al. [25] reported one case of a 7-year-old female patient who had about 20 nodules with varying sizes, the maximum one was 7 cm in diameter, which were confirmed as hepatic adenomatosis via liver biopsy. She was the youngest patient that has ever been reported. Most cases exhibit no clinical symptoms, though abdominal pain and fever can be found in cases with intratumoral hemorrhage, and severe pain, shock, and even death can be observed in cases with tumor rupture into the abdominal cavity. Imaging often shows multiple intrahepatic lesions consistent to the nature of hepatic adenoma.
6.1.2.3 Pathological Features
Histopathology of single nodule in hepatic adenomatosis is identical to that of simple hepatocellular adenoma , the relevant description of which has been discussed in the first section of this chapter.
6.1.2.4 Molecular Pathology
Iwen et al. [26] reported one case associated with juvenile patient with diabetes type 3 and hepatic adenomatosis who was found to have Q495X mutation, which was suggested to repress the expression of wild HNF1A gene, resulting in the genesis of the disease. His father had the same mutation but only suffered from impaired glucose tolerance, suggesting the necessity of liver examination for diabetes patients without autoimmune antibodies and oral glucose tolerance test (OGTT) for adenomatosis patients without diabetes. Furthermore, OGTT screening is beneficial in the early diagnosis of diabetes in the patients’ lineal relatives with HNF1A mutation.
6.1.2.5 Differential Diagnosis
Hepatic adenomatosis should be differentiated from multiple hepatic focal nodular hyperplasia , liver cirrhosis, liver metastasis of tumor, liver dysplastic nodules , and liver multiple well-differentiated hepatocellular carcinoma .
6.1.2.6 Treatment and Prognosis
Hepatic adenomatosis belongs to benign tumors with severe potential complications, such as malignant transformation, tumor rupture, and hemorrhage. At present, the treatment of the disease is still in discussion with much controversy. For patients in whom the tumor cannot be completely excised, surgical resection of huge lesion can be conducted to remove part of the lesions, relieve symptoms, and prevent complications, followed by close follow-up subsequently. And in suspected malignant cases or cases with significant symptoms, surgical resection can be adopted. There have been cases in which liver transplantation was adopted, with good therapeutic effects in most of these cases, while reports of deaths due to postsurgical complications or HCC in transplanted liver have also been discovered [27].
6.2 Bile Duct Tumors
Intrahepatic bile duct benign tumors and tumorlike lesions account for 1.9–2.4% of bile duct epithelial tumors. Although benign tumors of bile duct are relatively rare, they are occasionally cancerous and should be differentiated from malignant and metastatic tumors both clinically and pathologically. Therefore, it is necessary to give enough attention to them.
6.2.1 Bile Duct Adenoma
6.2.1.1 Pathogenesis and Mechanism
Intrahepatic bile duct adenoma (BDA ), also known as benign cholangioma, is a type of low-incidence bile duct benign lesions. However, the biological characteristics of BDA are still not clear. Currently, it is believed that BDA is a post-damage repair process, including inflammation due to injury of liver cells and reactive hyperplasia of small bile ducts, and it is not relevant to bile duct hamartoma or hepatic cysts. Another hypothesis of the genesis of BDA involves peribiliary glands and thus can be called peribiliary gland hamartoma, because both of them have the same immunohistochemistry phenotype. However, due to the finding of K-ras mutation in BDA cases, it is also regarded as a true-type tumor.
6.2.1.2 Clinical Features
The average age of the patients was 55 years old (1.5–99 years), with men accounting for 59.39% and without specific symptoms or signs, most of which are found by chance in examinations or abdominal surgeries for other diseases. Enhanced MRI images demonstrate mild arterial enhancement and continuous enhancement in portal venous phase, and the apparent diffusion coefficient shown on diffusion-weighted MRI is more than twice that of the background of liver parenchyma [28]. Eight cases of BDA diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, with an average age of the patients of 56 years old (37–74 years), including six males, and four accidental discoveries of BDA lesions were made in HCC surgeries which received resection during the operations.
6.2.1.3 Gross Features
The tumors are often solitary small nodules, accounting for 82.9%, while about 10% of the cases involve multiple lesions, predominantly located in the subcapsular region or can also be present in the deep liver parenchyma, white or gray, round or oval in shape, hard in texture, with clear boundaries and no true envelopes. The average diameter of the nodules in about 90% cases is 0.6 (0.5–1) cm or occasionally even up to 9.2 cm. The eight cases of BDA diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, were all solitary lesions with an average diameter of 0.98 (0.2–3.8) cm [29] (Figs. 6.13 and 6.14).

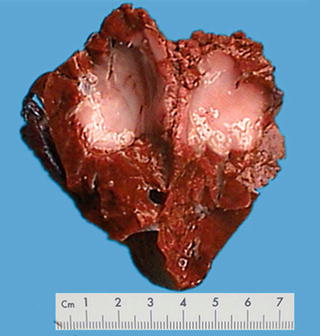

Fig. 6.13
Intrahepatic bile duct adenoma . A yellow round lesion with clear boundary and no envelope

Fig. 6.14
Intrahepatic bile duct adenoma . A gray-white round lesion with clear boundary, it is a canceration case
6.2.1.4 Microscopic Features
The lesions of BDA is composed of small bile ducts with similar sizes and narrow lumens, in a curved shape or a solid cords, mostly containing no concentrated bile (Fig. 6.15), showing no cystic cavity or dilation. The epithelial cells of the bile ducts are cuboidal or low columnar with no atypia and rich cytoplasm which is slightly basophilic, and the nuclei are small and round in uniform sizes showing no mitosis, even with visible focal mucinous metaplasia of glandular epithelium and moderate chronic inflammation mainly containing fibrous tissue and lymphocytes rather than ovarian interstitial tissue, and the fibrous interstitial tissue was loose and edematous or with hyaline degeneration. Residual normal bile ducts can be seen around the proliferated bile ductules in the portal area (Fig. 6.16), and no capsules are found surrounding the lesions while the boundary is clear showing no invasion into the surrounding liver tissue varying amounts of fat.
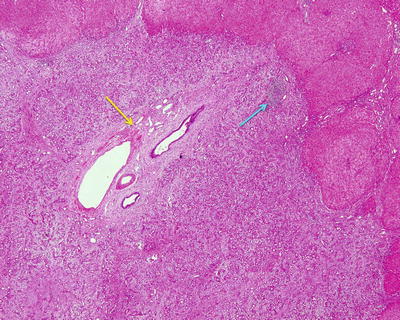

Fig. 6.15
Intrahepatic bile duct adenoma . Dense prolification of small bile ducts, remaining portal area, and focal lymphocyte infiltration (yellow arrow); the boundary is clear
Albores-Saavedra J et al. [32] and Wu et al. [30] reported four cases of clear cell-type BDA , 0.8–2.8 cm in diameter, with abundant clear cytoplasm in the tumor cells which arranged in shapes of small tubules, fine beam cable, or small nests, and more than 99% of the tumor cell cytoplasm was translucent, hyperchromatic nucleus were round or oval, and the substitution processes of normal bile duct epithelium by hyaline cells can been seen (Fig. 6.17).
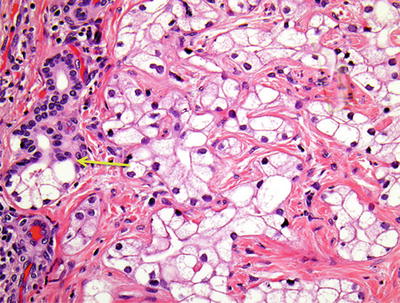

Fig. 6.17
Clear cell-type BDA . Substitution of normal bile duct epithelium by hyaline cells (yellow arrow)
Three cases of BDA with acidophilic degeneration in tumor cells have been reported [31, 33, 34], with the tumor diameter of 0.3–1.8 cm. Acidophilic degeneration refers to the generation of abundant fine granular eosinophilic cytoplasm in epithelial cells due to increase in the number of mitochondria under the microscope [33] (Fig. 6.18). The scarce bile duct tumors with acidophilic degeneration are mainly seen in intrahepatic bile duct papillary tumors and intrahepatic biliary cystadenocarcinoma , so whether BDA with acidophilic degeneration is a type of precancerous lesions for the aforementioned bile duct tumors should be discussed further. And comprehensive consideration on the nature of BDA should be made according to the size and cellular and nuclear morphology of the lesions.
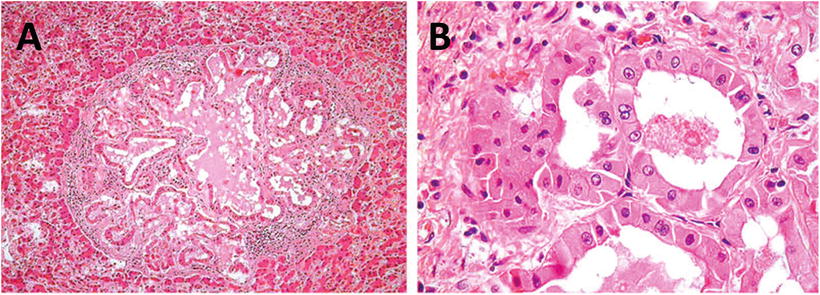

Fig. 6.18
BDA , with acidophilic degeneration. A,B: H&E staining showed abundant eosinophilic cytoplasm
Among the eight cases of BDA diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, four cases have concomitant HCC , one case with high-grade intraepithelial neoplasia, and one case canceration with transition between cancerous tissue and BDA (Fig. 6.19).
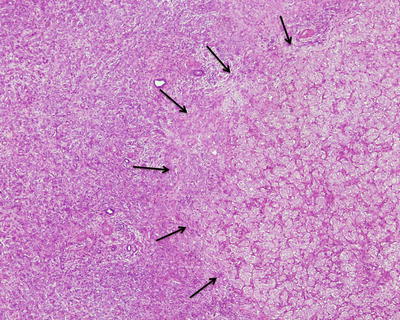

Fig. 6.19
Canceration with transition between cancerous tissue (right) and BDA (left)
6.2.1.5 Immunohistochemistry
EMA, CEA, CK, and CK19 (Fig. 6.20) staining are positive, while CK20 and hepatic cell marker staining should be negative. BDA also expresses CD10 and 1F6 antigen as peripheral glands do, and 1F6 is not expressed in the normal interlobular bile ducts. CD10 staining exhibits continuous stains on the luminal surface of the apical cells in the clear cell-type BDA and outlines the irregularly shaped lumen (Fig. 6.21).
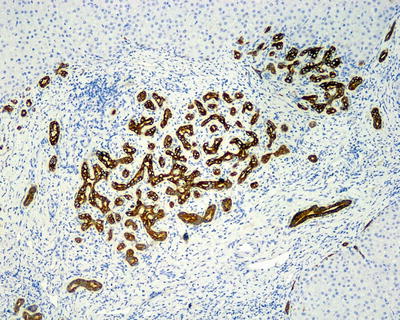
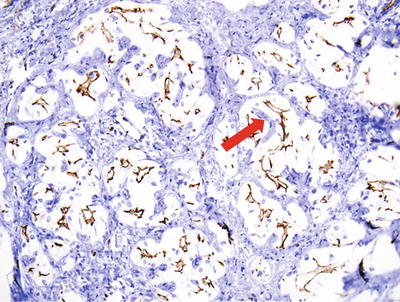

Fig. 6.20
CK19 immunohistochemical staining in BDA . CK19-positive expression in tumor gland duct (blue arrow), the normal interlobular bile ducts (red arrow)

Fig. 6.21
Clear cell-type BDA . CD10 staining exhibits continuous stains on the luminal surface of the apical cells in the clear cell-type BDA
6.2.1.6 Differential Diagnosis
BDA in liver parenchyma should mainly be differentiated from well-differentiated intrahepatic cholangiocarcinoma and metastatic adenocarcinoma, needing careful identification especially in the frozen biopsy. The author has encountered one case of a 71-year-old male patient receiving a surgical resection of gastric cardia adenocarcinoma, and a 0.2 cm gray-white nodule was found in the left lobe of the liver which was suspected as metastasis and excised during the operation. Later pathological examination confirmed is an intrahepatic BDA . Studies have shown that EZH2 and p16 (INK4a) can be used as markers of immunohistochemistry to distinguish ICC and BDA , because EZH2 is expressed in all ICC but not expressed in BDA , while p16 (INK4a) is expressed in 12% ICC and 81% BDA [36]. CD10 is expressed in benign tumors of bile duct but not expressed in malignant tumors, indicating that CD10 can be adopted as a marker for the identification of the nature of intrahepatic bile duct transparent cell tumors to tell whether a tumor is malignant or benign [37]. In addition, BDA and bile duct hamartoma are both benign intrahepatic bile duct proliferative lesions which are difficult to differentiate [35]. Main points of the differentiation for them are shown in Table 6.2.
Table 6.2
Differentiation of BDA and biliary hamartoma
Features | BDA | Biliary hamartoma |
|---|---|---|
Pathogenesis | Reactive hyperplasia of bile ducts | Development labyrinth of bile duct plates |
Number of nodules | Solitary | Multiple |
Diameter of nodules | >0.5 cm | <0.5 cm |
Texture of nodules | Solid | Cyst |
Bile duct epithelium | Cubic or short columnar | Flat or cubic |
Distribution of lesions | Random or beneath hepatic capsule | Surrounding or intra-portal area |
Cystic dilatation | None | Yes |
Arrangement of glandular ducts | Dense | Loose |
Bile in the lumen | None | Yes |
With polycystic disease | None | Yes in most of the cases |
Carcinogenesis | In some cases | In all cases |
6.2.1.7 Treatment and Prognosis
BDA grows slowly and may undergo hyaline degeneration to form scars. Despite the nature of benign lesions, canceration of BDA has already been reported in some cases. To date, BDA is recognized as precancerous lesions which may develop into bile duct carcinoma, so once diagnosed, surgical resection should be considered. For distal bile duct adenoma , endoscopic resection and argon plasma coagulation can be conducted for the treatment. And endoscopic resection in situ is suitable for the treatment of common bile duct adenoma .
6.2.2 Biliary Cystadenoma
6.2.2.1 Pathogenesis and Mechanism
Biliary cystadenoma (BCA) was reported by Hueter in 1887 for the first time, and Keen reported the first BCA resection in 1892. An epidemiological study published in 1993 has shown that 5–10% of the population may have cystic lesions in the liver, and the occurrence rate of bile duct cystic lesions was less than 5% the rate of intrahepatic cysts. And 90% bile duct cystic lesions arise in the intrahepatic while only 10% occur in extrahepatic biliary system.
The etiology of BCA remains unclear; Wheeler and Edmondson speculated that it may be a tumor formed during the development of ectopic bile ducts in the embryo. The patients may have hepatic cysts, polycystic liver abnormalities, or abnormal hepatic bile ducts. Comparison between mucous membrane of the gallbladder in adult BCA and fetus showed that the columnar epithelium in the former was similar to that in the embryonic development both under light microscope and electron microscope, mesenchymal vimentin staining was positive, and the latter only appeared in the fetal gallbladder tissue at 15th week of gestation, suggesting that BCA may derive from the ectopic embryonic gallbladder tissue. Another hypothesis for the genesis involves ectopic ovarian tissue or residual tissue of congenital embryonic foregut. In addition, due to endocrine cells found in about 50% of bile duct cysts, it has been also suggested that BCA may originate from intrahepatic peritubular glands.
6.2.2.2 Clinical Features
More than 85–90% of the patients are middle-aged or elderly women. An American report by Devaney et al. (1984) concerned a group of 52 cases of BCA patients, aged from 2 to 87 years old, and the average age was 45 years old, including 51 cases of mucinous type. Seventy-eight cases of BCA have been diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military University, with 55 cases of females (70.5%). The average age was 49.3 (26–77) years old, and the majority of the patients had no symptoms or only manifested nonspecific symptoms such as abdominal pain. Some patients came to the hospital because of abdominal tumor rupture and bleeding, secondary infection, obstructive jaundice, etc., and some had ascites as the first symptom due to rupture of BCA. Cases of BCA with pleural effusion have also been reported [48].
More than half of the patients have varying degrees of elevated serum CA19-9 [(838.4 + 2485.7) U/ml, reference value of 0–37 U/ml], mild elevation of serum alkaline phosphatase (ALP), γ-glutamyl transfer peptidase (γ-GT) and CEA, and negative serum AFP detection, all of which can turn normal after complete resection of the tumors. Despite controversy, cystic fluid obtained via puncture for tests of CA19-9 and CEA level were reported to be adopted to differentiate bile duct cystic lesions from cysts and abscess. However, due to the risk of rupture of the lesions leading to ascites or pleural effusion and the major difficulty in differentiation of benign tumor and malignant ones, cyst puncture biopsy should be conducted with much cautiousness.
ERCP cholangiopancreatography often shows filling defects [51]. B-ultrasound examination shows irregular thickening of the cyst wall and several thickened septa inside the cavity, with papillary projections and nodules on the inner wall of the cyst. CT images display several hypodense circular regions inside the cystic mass. And MRI images show typical irregular thick-walled multilocular lesions.
6.2.2.3 Gross Features
BCA occurs mainly in the liver, which can be located in the liver parenchyma, underneath the capsule, or even into the bile duct in the form of polyp, or involves both intra- and extrahepatic bile ducts, characterized by solitary multilocular cystic lesions (Fig. 6.22), and in some cases BCA are unilocular cysts with a smooth inner wall, while cases of multiple cysts are rare. Mucoid BCA contains transparent mucus or has a soapy section, and serous BCA is microcystic similar to cystadenoma of the pancreas. Both types of BCA can occur with intraductal papillary tumors. Among the 78 cases of BCA diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, 60 cases are multilocular, with an average diameter of 8.4 cm, and the maximum diameter was 34 cm in one case (Fig. 6.23). Sixty-nine cases concerned the intrahepatic bile duct, five cases extrahepatic bile duct, and four cases hilar bile duct, while one case involved both the left lobe of the liver and extrahepatic bile ducts. In cases with cystic papillary hyperplasia on the inner cystic wall, gray-white granular papilla can be observed, with clear, yellow-brown mucinous, jelly or bloody cystic fluid and complete fibrous capsules.
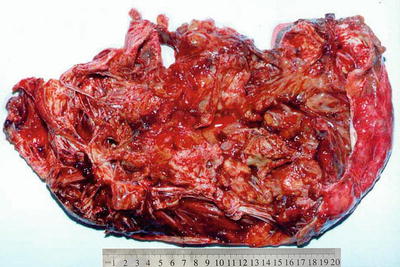
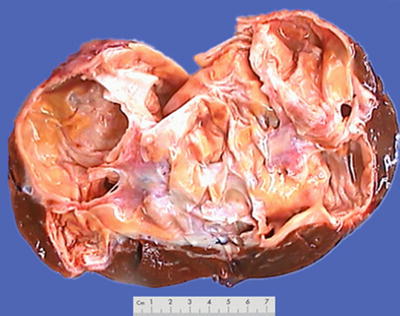

Fig. 6.22
Biliary cystadenoma . The tumor was huge, cystic, and solid

Fig. 6.23
Biliary cystadenoma . A multilocular cystic lesion
6.2.2.4 Microscopic Features
- 1.
Mucinous BCA. Columnar, cuboidal, or flattened epithelial cells are lined on the basement membrane of the cyst wall, which secretes mucus, with pale eosinophilic cytoplasm, nucleus located in the basal part, and no atypia (Fig. 6.24). Sometimes there will be focal intestinal epithelial metaplasia or absence of the lining epithelium (Fig. 6.25). The tumor cells were arranged into tubules. Bile duct papillary cystadenoma can be diagnosed according to the existence of papillary hyperplasia. Borderline lesions are indicated if dysplasia of epithelial cells is observed, such as increased nuclear volume, increased chromatin, multilayer arrangement, absence of cellular polarity, and mitotic activity. Lesions with highly graded atypical hyperplasia have a tendency of canceration, and junctional zone of normal mucosal epithelium-highly graded atypical lesion-cystadenocarcinoma can be found in canceration cases. Among the 78 cases of BCA diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, 30 cases involved low- or high-grade intraepithelial neoplasia, 3 cases involved canceration, and statistical analysis showed patients with portal or extrahepatic bile duct tumors, or male patients had higher proportions of intraepithelial neoplasia or canceration (Table 6.3). It is worth noting that two cases of hilar BCA had canceration. The cystic wall of BCA was composed of dense fibrous tissue with potential secondary changes, such as macrophages containing foam or pigments, hyaline degeneration, hemosiderin deposition, cholesterol fracture, focal calcification, etc. Spindle cells with round nucleus are found between the basement membrane and the lower part of the outer layer of fibrous connective tissue, which can go on differentiation to smooth muscle cells, fibroblasts, or adipose tissue and are termed as ovarian-type stroma. About 85% of mucinous BCA cases can have ovarian-type stroma, but only in females, while BCA cases with absence of ovarian-type stroma often concern male patients and only a few females. And among the 78 cases diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, 37 cases of ovarian-type stroma are all women.
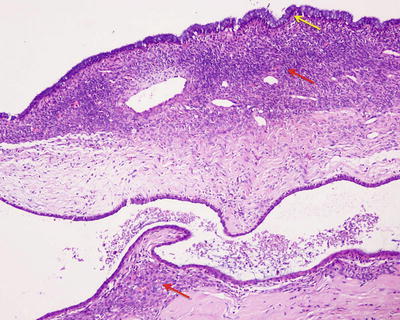
Fig. 6.24
Intrahepatic biliary cystadenoma . Cuboidal epithelial cells are lined on the surface of the cyst wall; the lower layer was the ovarian-type stroma
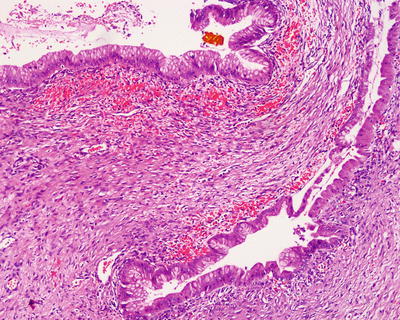
Fig. 6.25
Intrahepatic biliary cystadenoma . Intestinal epithelial metaplasia of the lining cells, ovarian-type stroma, and few hemorrhages were seen in the cyst wall
Table 6.3
Relationship of location and gender with intraepithelial neoplasia or carcinogenesis
Factors
Intraepithelial neoplasia or carcinogenesis
P value
Yes
No
Gender
Male
16
7
P=0.002
Female
17
38
Location
Extrahepatic or portal
7
2
P=0.022
Intrahepatic
26
43
- 2.
Serous BCA. The cyst wall is lined with a single layer of cuboidal epithelium with bright cytoplasm because of the rich glycogen, and mucous staining shows negative. The lower layer below the basement membrane is connective tissue with hyaline degeneration, and the interstitial tissue is composed of varying amounts of smooth muscle, fat, mucous glands, capillaries, and inflammatory cells rather than spindle cells.
6.2.2.5 Immunohistochemistry
Glandular epithelium was positive for CA19-9, low molecular weight CK, CEA, and EMA, similar to that of the normal bile duct and the pancreatic and ovarian tumors. Approximately 5% of BCA may have neuroendocrine differentiation; immunohistochemistry shows positive for synaptophysin (Syn) and chromogranin A (CgA) staining. Ovarian-type stroma was positive for vimentin, actin, desmin, and SMA, suggesting differentiation of smooth muscle, and expression of ER and PR, which may explain the reason of female predomination in BCA patients. Reports demonstrated that BCA often occurred in patients with hormone therapy, and tumor volume increased during pregnancy [53, 54].
6.2.2.6 Differential Diagnosis
- 1.
Differentiation should be made between BCA and various kinds of cystic lesions in the liver, such as bile duct hamartoma, cystic mesenchymal hamartoma , liver hydatid cysts, post-traumatic cysts, liver abscess, polycystic liver disease, bleeding cysts, embryonal sarcoma, HCC with cystic necrosis, metastatic tumors, biliary cystadenocarcinoma , teratoma , etc., all of which are without overlying epithelial ovarian-type stroma. Serum CA19-9 is synthesized by normal pancreatic and biliary ductal epithelium and thus will increase mildly in BDC which may be mistaken as a malignant tumor.
- 2.
Mucinous papillary BCA should be particularly distinguished from intraductal papillary mucinous tumors. Females account for the majority of all BCA patients, often with multilocular cysts and the mucous confined in the cystic cavity, while IPNB has no obvious gender difference in incidence, and the mucus can be found in all bile ducts. Both tumors are intraductal papillary neoplasms, demonstrating papillary protruding growth of epithelial cells from the cyst wall under the microscope; however, BCA cysts do not have communication with the normal bile duct system, while IPNB may involve multiple bile ducts and exhibit transition of bile duct epithelial cells. Microscopically, ovarian-like stroma can be found in BCA but not in IPNB . Furthermore, both have malignant potential, and IPNB is more prone to recurrence and residue due to crisp nature and easy defluvium of the tumor tissue and larger involvement [52].
- 3.
BCA and biliary cystadenocarcinoma are both rare liver-occupying lesions, but are common bile duct cystic lesions, with similar imaging manifestations and clinical manifestation which are difficult to distinguish. According to the studies by Sang et al. [49]. (2011) and Wang et al. [50], some clinical indicators can be used for the differentiation (Table 6.4).
Table 6.4
Clinical indicators for differentiation of BCA from bile duct cystadenocarcinoma
Indicators
BCA
Bile duct cystadenocarcinoma
Average age (years)
44.2
57
Gender ratio
Largely female (>85%)
Male and female (~1:1)
Average tumor diameter
13 cm
8.3 cm
Average serum CA19-9
838.4 U/ml
337.9 U/ml
6.2.2.7 Treatment and Prognosis
BCA grows slowly; however, up to 25% of the BCA may develop into biliary cystadenocarcinoma in a few years as pointed out by some scholars, and 33% of mucinous cystadenocarcinoma tissues contain residual benign cystadenoma tissue or transition of severe atypical epithelial hyperplasia and carcinoma tissue. Therefore, BCA is considered to be a precancerous lesion for biliary cystadenocarcinoma and should receive active surgical resection. In addition, ovarian-like stroma may also develop into cystadenocarcinoma or sarcoma [55–60].
The patients with complete resection of BCA have a good prognosis, but partial resection is potentially complicated by recurrent or residual cancer. Patients of about 132 cases reported in English literature during the recent 20 years of follow-up underwent surgical resection, with only 3 cases of recurrence after operation. There are reports on increased risks of recurrence in incomplete resection cases. Sun Zengpeng [61] reported 15 cases of BCA, with no recurrence in 12 cases receiving complete resection during the follow-up period, while patients with partial resection in 3 cases had recurrence 5 months, 12 months, and 18 months after the operations, respectively. Shen Hua [62] reported 11 cases of BCA, with 2 cases of canceration. One patient received puncture and drainage, developed recurrence and metastasis 2 years later, and died 3 months later, while the other patient was without recurrence. Therefore, puncture of liver cystic masses which is unclear in nature is with a considerable risk. Romagnoli et al. [63] reported one case of BCA with injury of intrahepatic bile duct, during the surgery due to the deep location of the tumor, and it was then treated via liver transplantation, obtaining satisfactory curative effect.
6.2.3 Intraductal Papillary Neoplasms of the Bile Ducts
According to the fourth edition of the WHO’s Neoplasms of Digestive System in 2010, intraductal papillary neoplasms of the bile ducts (IPNB ) include papilloma or papillary adenoma and papillomatosis, among which papillary tumors with mucous secretion are known as intraductal papillary mucinous biliary neoplasm (IPMN) , and multiple bile duct papillary tumors are called biliary papillomatosis.
6.2.3.1 Pathogenesis and Mechanism
With the gradually deepening knowledge on IPNB in recent years, more and more reports on the disease have been found, and there are more than 100 cases reported in the literature both abroad and at home since 1958 when the first case was reported by Caroli.
Studies from Taiwan, China, and South Korea showed that cholelithiasis and clonorchiasis are two major risk factors of this disease, which has highly malignant tendency. It takes 6–8 years for bile duct stones to develop into IPNB , while it takes 1–2 years for the development of a carcinoma into invasive cancer, the benign-to-malignant development of which is consistent with the general canceration process of adenoma, similar to the process of colorectal adenoma-adenocarcinoma. In addition, this tumor also involves inflammatory reaction, cell damage and repair, and dysplasia [64, 65]. IPNB often shares gene mutation spectrum with cholangiocarcinoma , including KRAS, TP53, p16, and SMAD4. It has been reported in the literature that 40–80% IPNB contain components of tubular adenocarcinoma, mucinous adenocarcinoma, or invasive carcinoma, indicating a high malignant risk for IPNB canceration. A total of 29 cases of IPNB have been diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, among which 27 patients are with low- or high-grade intraepithelial neoplasia or carcinogenesis.
The histogenesis of IPNB is still unknown and there are a variety of views. Nakanuma et al. [67] suggested that IPNB probably originated from peribiliary gland cells, while Cardinale et al. considered periductal stem or progenitor cells surrounding the bile duct as the origination, and the genesis includes a sequence of processes including reaction to risk factors such as inflammation, mutations in stem or progenitor cells, and gradual development of dysplasia into invasive cancer.
6.2.3.2 Clinical Features
According to an epidemiological survey in 2012 [66], the male to female ratio of IPNB patients was 1.06:1, with an average age of 60 (ranged 40–77) years old, and the majority of the patients are 50–70 years old. The most common clinical symptoms are recurrent abdominal pain, jaundice, and fever, and acute cholangitis is commonly seen in mucoid cases, while patients with non-mucoid IPNB are often asymptomatic. Some patients are complicated by hepatolithiasis, ulcerative colitis, Caroli disease, choledochal cyst, and polyposis coli. Twenty-nine cases have been diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, concerning 13 males and 16 females, with the average age of 59.7 (38–79) years old, including five cases with biliary stones and one case of colorectal cancer and liver metastasis. Imaging study demonstrates intraductal masses and dilatation of bile duct, and dilatation of both proximal and distal bile ducts has certain significance in diagnosis of IPNB . Laboratory examination results are often indicative of the general performance of the bile duct obstruction, such as an increase in ALT, AST, total bilirubin, conjugated bilirubin, γ-glutamyl transferase, and alkaline phosphatase. About 74.4% of the patients have elevated total serum bilirubin, 40–80% have elevated serum levels of CA19-9 which is much higher in mucous cases, and about 25% of patients have increased serum CEA.
6.2.3.3 Gross Features
IPNB usually consists of diffuse or multicentric papillary clusters which are ecru in color, connected to the bile duct mucosa via peduncles and mostly found in the left hepatic lobe, involving the whole biliary tree or even the extrahepatic bile ducts, cystic duct, and main pancreatic duct. The tumor, crisp, soft, and easy to fall off, mainly generated from epithelial growth on the surface, protruding into the lumen, and may cause complete obstruction of the bile ducts. The surrounding liver tissue is green in color due to cholestasis or presents biliary cirrhosis. Among the 29 cases of IPNB diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, 19 cases involved intrahepatic bile ducts, and 10 cases involved the hilar or extrahepatic bile ducts. The cross section of the tumors exhibited obvious dilatation of the bile ducts filled with gray-white or gray-red floccule, tumor thrombus-like tissue (Fig. 6.26), bile duct polyp, or cauliflower mass in the lumen.
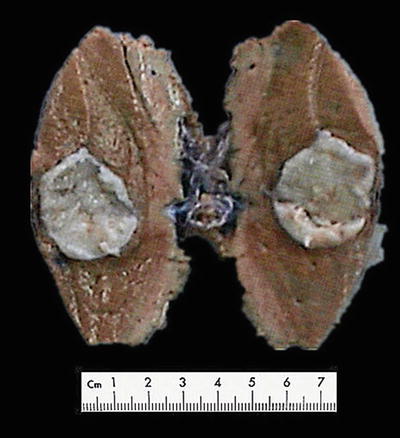

Fig. 6.26
Intraductal papillary neoplasms of the bile ducts. The bile ducts filled with gray-white polyp tissue
6.2.3.4 Microscopic Features
Histologically, IPNB shows multiple papillary-villous hyperplasia of biliary epithelium, which grows in branches from fibrous vascular axis protruding into the lumen and lined with cuboidal or columnar epithelium on the surface. The cells are with pale eosinophilic cytoplasm (Figs. 6.27 and 6.28) and arranged in multilayers with darker staining nuclei during dysplasia (Figs. 6.29 and 6.30). And as the degree of dysplasia increases, the cell layers and cells with mitosis multiply, with a visible transition between the lesion and the epithelium of normal bile duct (Fig. 6.31). The bile ducts dilate significantly, surrounded by normal bile ducts with acute or chronic inflammation of epithelium, which may detach due to ulcer, and duct wall thickens because of fibrous tissue hyperplasia. Most of the lesions protrude into the lumen which may be filled up, and infiltration into basal bile duct wall can be observed when canceration occurs (Fig. 6.32). Mucus secretion is found in one third cases of IPNB (IPMN ). IPNB is prone to be malignant; thus, multiple sections are needed for careful examination. According to different degrees of dysplasia and infiltration, it can be divided into four stages: IPNB with low-grade intraepithelial neoplasia; IPNB with high-grade intraepithelial neoplasia; intraductal carcinoma of bile duct in situ, T1; and infiltrating carcinoma of the bile duct, T2 or higher. The 29 cases of IPNB diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, included 8 cases with low-grade intraepithelial neoplasias, 14 cases with high-grade intraepithelial neoplasia, and 5 cases with canceration.
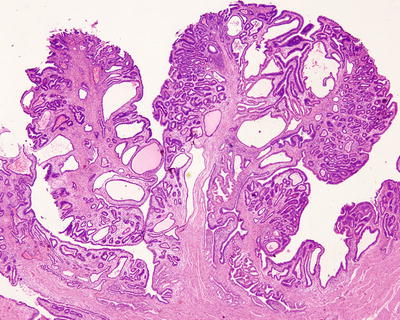
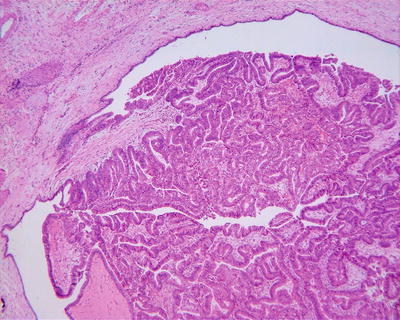
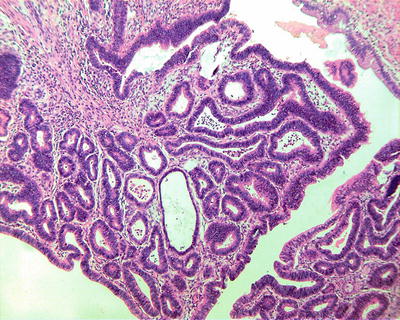
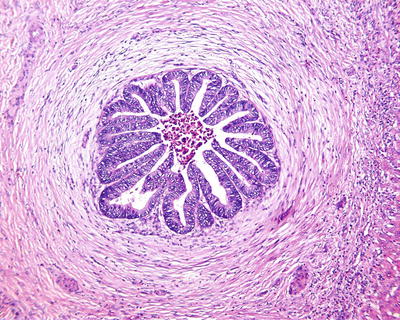
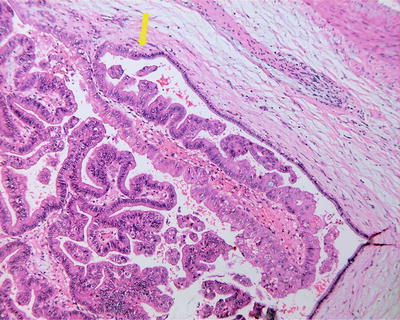
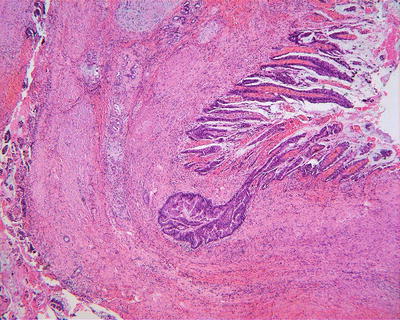

Fig. 6.27
Intraductal papillary neoplasms of the bile ducts. IPNB shows papillary hyperplasia, which grows in branches from fibrous vascular axis protruding into the lumen

Fig. 6.28
Intraductal papillary neoplasms of the bile ducts

Fig. 6.29
Intraductal papillary neoplasms of the bile ducts with high-grade intraepithelial neoplasia. The tumor cells are arranged in multilayers with darker staining nuclei. High power of Fig. 6.28

Fig. 6.30
Intraductal papillary neoplasms of the bile ducts with high-grade intraepithelial neoplasia. The small bile duct branch cells are arranged in multilayers with darker staining nuclei; the lumen is narrow with necrosis

Fig. 6.31
Intraductal papillary neoplasms of the bile ducts. Transition between the lesion and the epithelium of normal bile duct

Fig. 6.32
Intraductal papillary neoplasms of the bile ducts with canceration. Papillary hyperplasia of biliary epithelium infiltration into basal bile duct wall
According to the tumor tissues and the morphological characteristics, IPNB can be divided into the following four types:
- 1.
Pancreatobiliary type. The tumor is similar to the pancreas duct and ductular epithelium with columnar cells which have basophilic cytoplasm and round nuclei (Fig. 6.33). Immunohistochemistry staining often shows MUC1 positive and MUC2 negative. It is the most common type which tends to undergo malignant transformation to form tubular adenocarcinoma.
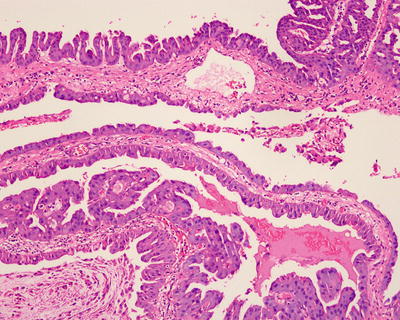
Fig. 6.33
Intraductal papillary neoplasms, pancreatobiliary type. The tumor is similar to the pancreas duct and ductular epithelium with columnar cells
- 2.
Intestinal type. The tumor has similarity to the features of intestinal villous tumors (Fig. 6.34), and immunohistochemistry staining shows that the tumor cells stably expressed MUC2 and MUC5AC, while no expression of MUC1 was detected. It is less likely to undergo malignant transformation which results in colloid (mucinous) invasive carcinoma.
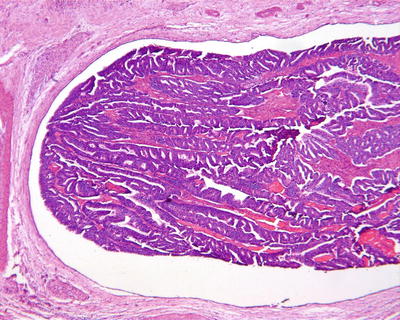
Fig. 6.34
Intraductal papillary neoplasms, intestinal type. Columnar tumor cells have similarity to the features of intestinal villous tumors
- 3.
Oncocytic type. The tumor cells have rich cytoplasm which is strongly eosinophilic (Fig. 6.35), and immunohistochemistry staining shows stable expression of MUC5AC and focal expression of MUC1 and (or) MUC2 in the cells. It has less tendency of malignant transformation.
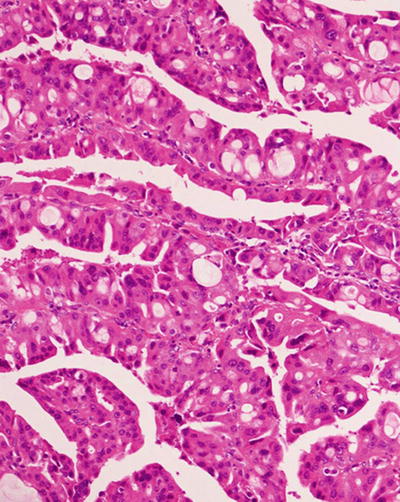
Fig. 6.35
Intraductal papillary neoplasms, oncocytic type. The tumor cells have rich cytoplasm which is strongly eosinophilic
- 4.
Gastric type. The tumor consists of columnar epithelial cells, similar to the gastric pit structure (Fig. 6.36), and immunohistochemistry staining shows MUC5AC expression and no expression of MUC1 or MUC2.
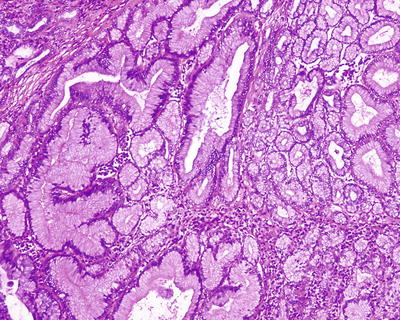
Fig. 6.36
Intraductal papillary neoplasms, gastric type. The tumor consists of columnar epithelial cells, similar to the gastric pit structure
Papillomatosis was reported by Caroli in 1959 for the first time, and more than 150 cases have been reported till now. The lesions grow in multiple foci, with involvement of the whole intrahepatic biliary tree in severe cases, and both intra- and extrahepatic bile ducts can be involved. Furthermore, lesions in different regions may be at different stages in the developmental course and can be divided into mucus biliary papillomatosis (MBP) and non-mucus biliary papillomatosis (NMBP). Generally, it is believed that BP has a highly malignant tendency and is an important type of precancerous lesions . Its canceration rate is as high as 41–83%, and radical surgical resection or liver transplantation should be preferred for the treatment [68].
6.2.3.5 Immunohistochemistry
Almost all of the IPNB lesions express biomarkers of bile duct epithelium and gastrointestinal epithelium, such as CK7, CK20, and MUC5AC, suggesting the combination of its retaining bile duct epithelial immunophenotypes and obtaining MUC1 immunophenotypes of the gastrointestinal epithelium in the genesis of IPNB . The expression of MUC1 usually indicates the infiltrating carcinoma, while mucinous tumors in IPMN are MUC1 negative and MUC2 positive. Sasaki et al. [69] demonstrated that IPNB overexpressed EZH2 and MUC1, and the low expression of MUC6 may indicate the malignant biological behavior of the tumor. Tubulin β-III (TUBB3) is negative in IPNB but positive in about half the number of extrahepatic bile duct carcinoma. Murakami et al. [70] found P53, CD133, and mucus staining were positive in primary tumors and cell line (KBDC)-11 of IPNB .
6.2.3.6 Differential Diagnosis
IPNB should be distinguished from intrahepatic bile duct stones, bile duct cystadenoma or carcinoma, cholangiocarcinoma , etc.
- 1.
Intrahepatic bile duct stones. The clinical symptoms of intrahepatic bile duct stones are similar to that of IPNB , and the latter can be complicated by bile duct stones, while intrahepatic bile duct stones often present simple dilatation of the bile duct, active proliferation of the peripheral glands, interstitial infiltration of lots of inflammatory cells, no papillary hyperplasia of epithelium, and little mucus in the lumen under the microscope. They are benign lesions, but intraepithelial neoplasia and cancer can also be found occasionally. Therefore, careful sample drawing is needed to prevent missed diagnosis.
- 2.
Biliary cystadenoma . IPNB is without ovarian-like stroma, and its cystic dilatation is communicated with bile ducts.
- 3.
Cholangiocarcinoma . Cholangiocarcinoma exhibits no cholangitis or biliary stones in most cases, and visible mucus is not common in it, while the above three manifestations are common in IPNB . Enhancement of IPNB is noted in outflow phase of enhanced CT, rather than persistent or progressive enhancement as in cholangiocarcinoma .
6.2.3.7 Treatment and Prognosis
IPNB may develop into intrahepatic papillary carcinoma or mucinous cholangiocarcinoa and is a defined type of precancerous lesions . Focal resection is for limited localized lesions in one side of the hepatic lobe. However, lesions that are widely distributed cannot be easily removed, and they are often crisp and easy to fall off, resulting in high relapse rate after surgery and poor prognosis. Papillomatosis can also cause a variety of complications, including intrahepatic duct stones, biliary hemorrhage, common bile duct stones, and gallbladder stones. Other common and fatal complications include bacterial cholangitis, obstructive jaundice, sepsis, hepatic failure, and malignant transformation, which need liver transplantation when necessary.
Generally, the growth of IPNB is mainly limited inside the bile duct, and it has a better prognosis than that of common intrahepatic cholangiocarcinoma . Mucous IPNB accounts for 83% of all the cases, and the average postoperative survival period of non-mucinous IPNB is reported longer than that of mucous IPNB , 52.27 + 6.72 months and 30.84 + 8.36 months, respectively. However, biological behaviors of IPNB lesions differ from each other due to their different stages. Rocha et al. [71] demonstrated that different infiltration depths and different canceration degrees of the tumors are influencing factors for patients’ survival time. Kim et al. [72] suggested that the biological behavior of pancreatobiliary type was different from other types with a higher risk of lymph node metastasis and recurrence.
6.2.4 Biliary Adenofibroma
Wen-Ming Cong5 and Yu-Yao Zhu5
(5)
Department of Pathology, Eastern Hepatobiliary Surgery Hospital,, Second Military Medical University,, Shanghai,, China
6.2.4.1 Pathogenesis and Mechanism
Biliary adenofibroma (BAF) is a very rare tumor, first reported by Tsui et al. in 1993 [73], and is considered as a benign tumor with potential of malignant transformation originating from the bile duct. Only six cases of BAF have been reported in the Chinese and foreign literature (Table 6.5), and one case was diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University. Concentrated bile was found in the duct of BAF indicating the connection to the bile duct system, which is a supportive evidence that it derives from hamartomatous bile ducts. Parada et al. [74] detected the chromosome mutation of one case of BAF using cytogenetic methods, finding abnormal chromosome 22, which is common in benign mesenchymal tumors, suggesting that BAF is originated from mesenchymal tissue rather than epithelium.
Table 6.5
Reported cases of biliary adenofibroma
Authors | Year | Gender | Age (years) | Diameter (cm) | Malignancy | Recurrence/metastasis | Feature |
|---|---|---|---|---|---|---|---|
Tsui et al. [73] | 1993 | Female | 74 | 7 | None | None | Right upper abdominal pain |
Parada et al. [74] | 1997 | Female | 49 | 7.5 | None | None | Right hypochondrium pain |
Akin and Coskun [75] | 2002 | Male | 25 | 20, recurrent 14 | Yes | Recurrence 3 years postsurgery/lung metastasis | Abdominal distension and right upper abdominal pain |
Varnholt et al. [76] | 2003 | Female | 47 | 16 | None | None | Right upper abdominal pain for several months |
Xu Li et al. [77] | 2009 | Female | 65 | 3.5 | None | None | Intermittent right upper abdominal pain for 10 years |
Alessandra et al. [78] | 2010 | Male | 79 | 5.5 | None | None | Dull abdominal pain |
6.2.4.2 Clinical Features
The average age of the patients is 56.5 (25–79) years old, and the first symptom is often dull pain in the right upper abdomen, with normal laboratory examination and hypervascular masses in CT scanning. The case diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, concerned a 51-year-old patient, who was admitted into the hospital because of upper abdominal pain for 5 months and an occupying lesion in the right lobe of the liver found in CT examination, with normal laboratory examination and no history of hepatitis.
6.2.4.3 Gross Features
The tumor is a spherical mass with an average diameter of 9.9 (3.5–20) cm, and the section shows multiple closely distributed oval thin-walled cavities with a diameter of 1–5 mm accounting for three fourths of the volume of the tumor. The cystic cavity was divided into lobules by slender septa throughout the tumor, and the remaining part of the tumor is composed of relatively dense stroma. The tumor has a clear boundary with no capsule and paler than the surrounding liver tissue in color, while the latter may exhibit atrophy. One case of BAF was diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, the size of which was 3 cm ×2.4 cm × 1.3 cm, the cross section gray and grayish yellow, and hard in texture, with visible fibrous bands, no hemorrhage or necrosis, multiple small cavities in the lesion, fiber bundles stretching out along the duct wall, no complete capsule, and a clear boundary (Fig. 6.37).
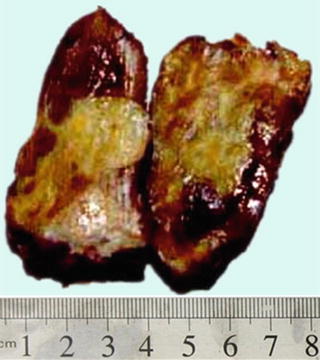

Fig. 6.37
Biliary adenofibroma. The cross section gray and grayish yellow, and hard in texture, with visible fibrous bands
6.2.4.4 Microscopic Features
The tumor consists of small vesicles and tubular or cystic cavity-like structures of varying sizes in loose and separated arrangement, embedded in abundant fibrous stroma (Fig. 6.38). The major cells of the tumor are cuboidal and columnar epithelial cells containing abundant cytoplasm with no atypia, and the cytoplasm of some epithelial cells is both eosinophilic and basophilous. The nucleus is small and round or oval, with small and clear nucleolus. The dilated ducts can be cystic with branches or bending, or small papillary protrusions formed on the ductal wall, and a few ducts contain cell debris, bile embolus, or thin eosinophilic fluid. The epithelial cells do not contain mucus, but can go through apocrine glands which changes occasionally on the inner layer of the ductal wall (Fig. 6.39), or are arranged in multilayers with darker nuclear staining and mild to moderate atypical hyperplasia. The interstitial fibrous tissue contains moderate numbers of cells which are myofibroblast-like and spindle shaped, with sparse and red-dye cytoplasm. The local interstitial tissue can exhibit inflammatory reaction mainly composed of lymphocytes, and densely distributed glandules can be found in sparse interstitial regions, similar to the features of BDA . Residual liver cell islands can be observed in the tumor tissue, with no envelope surrounding the tumor, a clear boundary, and no invasion into the surrounding liver tissue.
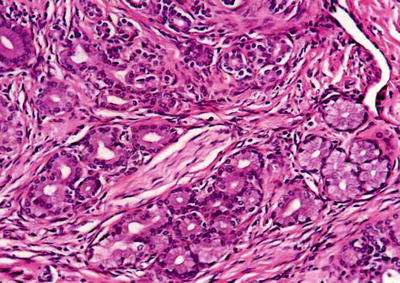

Fig. 6.38
Biliary adenofibroma. The tumor consists of proliferated small bile duct and abundant fibrous stroma

Fig. 6.39
Biliary adenofibroma. Abundant bile ducts and glands hyperplasia, with abundant collagen fibrous stroma
6.2.4.5 Immunohistochemistry
The epithelial cells are CK5.2, CK7, CK19, EMA, CEA and D10 positive, 1F6, desmin, SMA, mucus staining negative, and interstitial VI and SMA positive. P53 staining positive suggests potential for malignant transformation. Interstitial fibrous tissue staining demonstrates positive VI and SMA and negative NSE and S-100.
6.2.4.6 Differential Diagnosis
- 1.
Bile duct adenoma parts of the lesions may be similar to BDA , but BDA does not contain any bile, and it often presents tubular lumen of bile ducts rather than cystic cavities, which is narrow, closely arranged, and compressed by the matrix. BDA is usually 1–20 mm in diameter, and positive D10 and 1F6 are shown in immunohistochemistry staining positive.
- 2.
Biliary cystadenoma (BCA) is often a huge multilocular cystic mass, and the BAF sac and BCA are similar, but a BCA lesion is generally a solid one, less than 5 mm in diameter. And epithelial mucus staining is positive in BCA but negative in BAF, and the latter does not contain ovarian-like stroma.
- 3.
Well-differentiated cholangiocarcinoma , congenital choledochal cysts, liver benign cystic mesothelioma, and biliary hamartoma should also be taken into consideration during differentiation.
6.2.4.7 Treatment and Prognosis
BAF with positive p53 staining has been reported, presenting tetraploid and S phase block, suggesting that it may belong to the precancerous lesions . And if untreated, the epithelial cells in BAF can go on malignant transformation, especially in regions with multilayers of epithelial cells and darker staining. Therefore, complete surgical resection is the recommended treatment. Lesions with no atypia and negative p53 indicate benign and inert biological behaviors; however, there was one reported case of BAF with epithelial malignant components. Therefore, it is necessary to pay attention to the disease.
6.3 Vascular and Lymphatic Tumors
6.3.1 Cavernous Hemangioma
Wen-Ming Cong6 and Qian Zhao6
(6)
Department of Pathology, Eastern Hepatobiliary Surgery Hospital,, Second Military Medical University,, Shanghai,, China
6.3.1.1 Pathogenesis and Mechanism
Cavernous hemangioma is a benign vascular tumor composed of thin-walled honeycomb vascular cavities and is the most common benign tumor of the liver, with an incidence rate of 1–7%, accounting for about 74% of benign hepatic tumors. The tumor usually occurs in childhood and is often diagnosed in adulthood, regarded as a type of congenital lesions and related to vascular developmental loss of embryonic liver, or an acquired disease induced by steroids, contraceptive, pregnancy, etc. Recent studies have shown that accumulation of mast cells may be associated with its occurrence. From January 1982 to July 2014, 2732 cases of cavernous hemangioma have been diagnosed in the Pathology Department of East Hepatobiliary Surgery Hospital of Second Military Medical University.
6.3.1.2 Clinical Features
Among 172 cases of excised liver hemangioma , diagnosed in the East Hepatobiliary Surgery Hospital of Second Military Medical University from 2004 to 2006, the ratio of male to female ratio was 1:2.4, with an average age of 44.5 years old. Among them, 83 cases had symptoms, including 68 cases of abdominal pain, 16 cases of back and shoulder discomfort, and 4 cases of abdominal distension. The tumors can be palpated in the hypochondrium region in 25 patients, and the size of tumor increased significantly in 84 cases during the course of the disease, with an average increase of 4.4 cm. Most liver hemangiomas did not cause obvious symptoms or signs despite huge volume and were found only in the physical examination. Some of these patients may have symptoms, mainly chronic abdominal dull pain and postprandial fullness, which were occasionally mistaken as hepatic neoplasms. B-ultrasound examination showed small hemangioma with low to medium echo, and CT images showed hypodensity lesions, while MRI T2-weighted images showed high signal density, the so-called bulb sign.
6.3.1.3 Gross Features
Among 172 cases of excised liver hemangioma , diagnosed in the East Hepatobiliary Surgery Hospital of Second Military Medical University from 2004 to 2006, solitary lesions were found in 94 cases [79], two lesions in 40 cases, and three or more lesions in 38 cases. The tumors were 4–32 cm in diameter, averaged at 10.5 cm. A giant hepatic cavernous hemangioma was found and excised by Mengchao Wu and colleagues in one case with a tumor of 63 cm * 48.5 cm * 40 cm in size and 18 kg in weight. The cavernous hemangioma exhibited expansive growth, lobulated surface, and purple-red or dark red in color, soft, with streak-shaped fibrous coating. The cross section showed spongy or cellular lacunae containing blood components, often accompanied by varying sizes of gray-white fibrous sclerosis nodules. A few cases of hemangioma were found with degeneration, thrombosis, or further fibrosis and calcification, with gray fibrosclerosis nodules, and the hemangioma was called sclerosed hemangioma. Liver cirrhosis in the surrounding liver tissue was also found in patients with chronic hepatitis (Figs. 6.40 and 6.41).
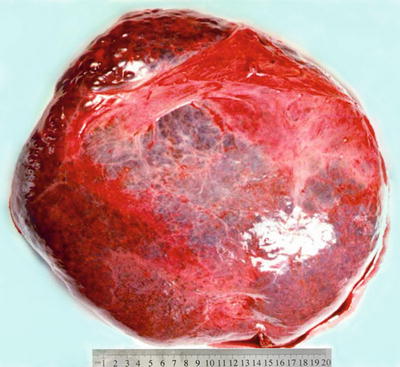
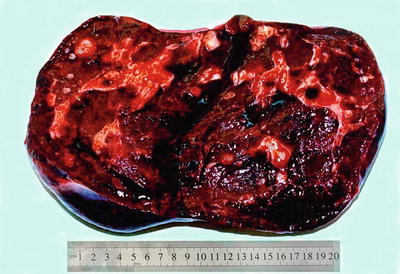

Fig. 6.40
Cavernous hemangioma . The tumor is purple-red in color and soft

Fig. 6.41
Cavernous hemangioma . The cross section showed spongy, accompanied by gray-white fibrous sclerosis nodules
6.3.1.4 Microscopic Features
Lesions of cavernous hemangioma are often uniform, composed of varying sizes of communicating vascular cavities, lined with flattened epithelial cells supported by fibrous tissue on the inner wall of the vessels, and filled with blood, and the vascular wall is often thickened to varying degrees due to fibrosis, also known as sclerosed hemangioma (Figs. 6.42, 6.43, and 6.44). The surrounding liver tissue is often distributed with scattered hemangioma foci or highly dilated vascular clusters.
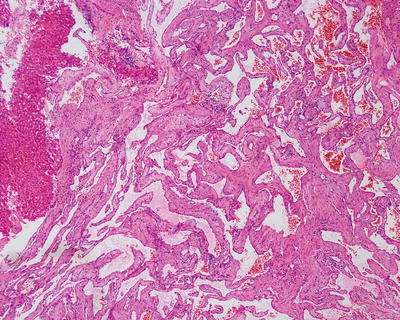
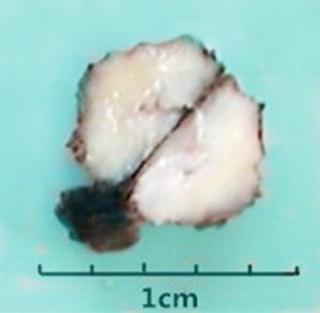
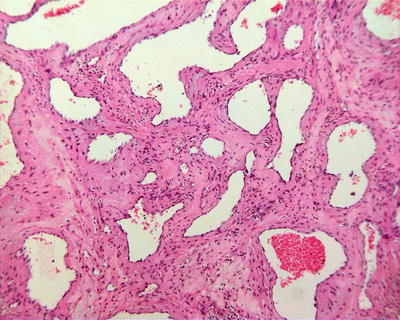

Fig. 6.42
Cavernous hemangioma . The tumor was composed of highly dilated thin-walled vessels, filled with blood

Fig. 6.43
Sclerosed hemangioma

Fig. 6.44
Cavernous hemangioma , sclerosed type. The vascular wall is thickened to varying degrees due to fibrosis
Hepatic hemangioma includes the following rare subtypes. ① Capillary hemangioma, diagnosed in a 58-year-old woman with a surgical resection of a tumor in the left lobe of the liver in our experience, 2.3 cm × 2 cm in size with a dark red section (Fig. 6.45), was clustered with immature capillaries (Fig. 6.46), lined by a monolayer flattened endothelial cells (Fig. 6.47) under the microscope, and CD34 staining showed the immature microvascular lumens (Fig. 6.48). In addition, visible thrombosis and arteries with thickened vascular wall and stenosis can also be seen. ② Infantile hemangioma (IH), also known as infantile hemangioendothelioma, often occurs in 10-day-old to 3-month-old infants after birth, with multiple lesions of infantile skin hemangiomatosis, 0.8–21 cm in diameter, and can be divided into focal (single lesion), multifocal (4–20 lesions), and diffuse type (>20 lesions). The morphological characteristics are similar to those of juvenile capillary hemangioma in the soft tissue. In the early stage of the disease, the neocapillaries may contain no or only a few cavities, and the hypertrophic endothelial cells are arranged in the shape of solid plates, while in maturity stage they are similar to adult capillary hemangioma with intensive expression of glucose transporter (GLUT1) and formation of angiosarcoma in a small number of cases [80], and the solitary lesions can be surgically excised, while multiple lesions can be treated by liver transplantation. ③ Hepatic vascular malformation with capillary proliferation (HVMCP), with lesions of 5–11 cm in diameter, typically exhibits as major infarction and hemorrhage center and strip-shaped arrangement of congested blood vessels with thick walls, lined with flat endothelial cells, surrounded by proliferation of peripheral blood capillaries and interstitial mucinous degeneration. Negative GLUT1 staining is shown in vascular endothelial cells and major blood vessels. The prognosis of the tumor which receives surgical resection is good [81].
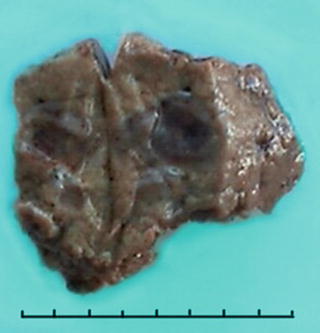
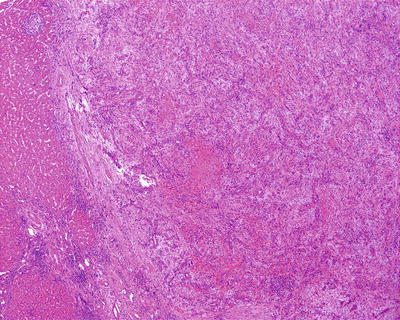
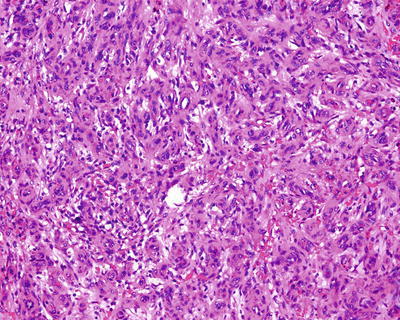
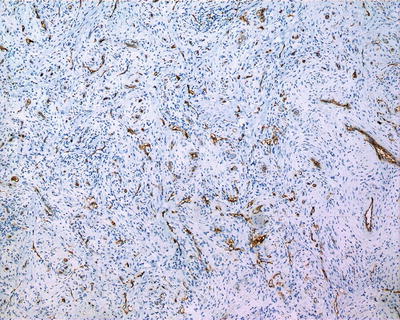

Fig. 6.45
Capillary hemangioma . The cross section showed dark red with clear boundary

Fig. 6.46
Capillary hemangioma . The tumor was clustered with capillaries; vascular lumens were narrow

Fig. 6.47
Capillary hemangioma . Immature small vessels lined by endothelial cells showed microvascular lumens

Fig. 6.48
Capillary hemangioma . CD34 staining showed the immature microvascular lumens
6.3.1.5 Immunohistochemistry
CD34 and F-VIII positive.
6.3.1.6 Differential Diagnosis
Pathological diagnosis of the disease is not difficult, but misdiagnosis from vascular lesions, such as angiolymphoma, angiosarcoma , and peliosis hepatis, should be avoided.
6.3.1.7 Treatment and Prognosis
Cavernous hemangioma grows slowly with no malignant tendency. Lesions >5 cm in diameter or manifests by obvious symptoms can be treated with surgical resection or hepatic artery ligation, and conservative treatment or observation is suited for small or asymptomatic lesions, while selective hepatic portal occlusion and anatomic technique are extremely important when treating hemangioma located at complex and difficult sites [82]. Successful liver transplantation was reported in a case of giant hepatic cavernous hemangioma (40 cm×30 cm×30 cm) by Zhongshan Medical University. In recent years, interventional technique and radiofrequency ablation have also been applied to the treatment of this disease which shows good curative effects in surgery. The prognosis is good, with no recurrence, and hepatic cavernous hemangioma associated with medication may disappear spontaneously or stop growing or after drug withdrawal.
6.3.2 Hemangioblastoma
Wen-Ming Cong7 and Yu-Yao Zhu7
(7)
Department of Pathology, Eastern Hepatobiliary Surgery Hospital,, Second Military Medical University,, Shanghai,, China
Hemangioblastoma, also called as haemangioblastomas and composed of large thick-walled blood vessels and stromal cells with rich lipid droplets, is a benign vascular tumor originated from mesoderm and commonly found in patients with von Hippel-Lindau disease (VHL). VHL is an autosomal dominant cancer predisposition syndrome, mainly involving multiple organs and systems and a variety of hereditary neoplastic syndromes, and hemangioblastoma of central nervous system is the most common tumor, often found in the cerebellum, cerebrum, spinal cord, brain stem, and retina and rarely seen in the liver. Only three cases of liver hemangioblastoma have been reported in the English literature, all of which were combined with VHL [83–85].
Previous reports of VHL involving the liver were often adenoma, cysts, and hemangioma , while liver hemangioblastoma is extremely rare. Ultrasound examination shows a solid hypoechoic mass, and CT shows a hypodense mass, while angiography shows a mass with rich blood vessels. Microscopic observation shows the similarity of the lesion to hemangioblastoma of the central nervous system, consisting of vascular endothelial cells, pericytes, and stromal cells which form atypical capillaries with expansion of vessels and a small amount of red blood cells in the lumen. The stromal cells are rich in adipose tissue, which may form bubbles or bubble-like structures, and increased nuclear chromatin and enlarged nuclei are occasionally found in the stromal cells. When VHL patients are accompanied with liver occupation, hemangioblastoma should be first excluded. Surgical treatment for intracranial hemangioblastomas shows good therapeutic effects, and complete resection is a radical therapy. However, there are also many reported cases concerning hemangioblastoma in the liver or lung after multiple surgeries.
6.3.3 Infantile Hemangioendothelioma
Wen-Ming Cong8 and Qian Zhao8
(8)
Department of Pathology, Eastern Hepatobiliary Surgery Hospital,, Second Military Medical University,, Shanghai,, China
6.3.3.1 Pathogenesis and Mechanism
Infantile hemangioendothelioma (IHE) is the most common benign vascular tumor in the liver; almost all cases concern infants under 1 year old after birth, derived from abnormal development of the central vein and portal vein system. From January 1982 to December 2012, six cases of IHE were diagnosed in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, of Second Military Medical University, accounting for 0.019% of all liver primary malignant tumors during the same period. IHE is similar to skin capillary hemangioma that if regression occurs after proliferation, maturation, and degradation stages, liver-occupying lesions cannot be formed. This disease can also be a part of Kasabach-Merritt syndrome, characterized by cutaneous and visceral angiomatosis, thrombocytopenia syndrome, and disseminated intravascular coagulation, or accompanied by certain congenital diseases, such as congenital heart disease, 21 trisomy syndrome, deletions in chromosome 6q, and heterotopic left liver in the thoracic cavity. Rapini et al. [86] conducted karyotype analysis in one 3-year-old child with IHE (tumor size, 57 mm × 56 mm × 70 mm), finding interstitial deletion of 13q13.3 to 21.32 on the long arm of chromosome 13, with a deletion range of 27.87 MB containing the RB1 gene, and the patient manifested developmental delay and poor growth.
6.3.3.2 Clinical Features
The patients of IHE include infants within 6 months after birth accounting for 86% and children under the age of 3 years old accounting for 99%. However, there are also reports of adult IHE. Among the six cases of IHE in the Department of Pathology, Eastern Hepatobiliary Surgery Hospital, of Second Military Medical University, the male to female ratio was 5:1, and the average age was 38.8 years (5–61). About 30% of the children cases had angioendothelioma and at the same time in the skin, lymph node, spleen, gastrointestinal tract, pleura, prostate, lung, and bone. Riley et al. [87] reported two cases of IHE with biliary atresia, acute liver failure, and some other congenital diseases, such as diaphragmatic hernia, Down syndrome, large artery transposition, and multi-finger deformity. Forty percent of the patients were primarily diagnosed when their mother manifested abdominal masses. Related clinical manifestations include hepatomegaly, nausea, vomiting, gastrointestinal bleeding, jaundice, hemolytic anemia, thrombocytopenia, liver failure, or even death caused by high-output heart failure because of intrahepatic arteriovenous shunt due to tumor compression. Mild to moderate increase of serum AFP levels can be found in the children patients, which is limited for reference in younger children, because its level in normal newborns reaches 2500 ng/ml and decreases to normal level in adults at 6 months after birth. The performance of the lesions on CT shows hypodensity with a clear boundary, and scattered calcification in the center can be found in 50% cases, while obvious calcification demonstrates wide distribution inside the mass-forming granular clusters. And early filling of the lesions in SPECT scans is of diagnostic value.
6.3.3.3 Gross Features
Intrahepatic solitary or multiple lesions are found in liver IHE. In our hospital, all the six cases diagnosed as IHE concerned solitary lesions of 1.2–6.3 cm in diameter. The cross section of the tumors was brownish-red capillary cavities with rich blood contents or yellow white in cases with necrosis. The boundary was not completely clear between the tumor and the surrounding liver tissue, and local infiltration can be observed (Fig. 6.49).
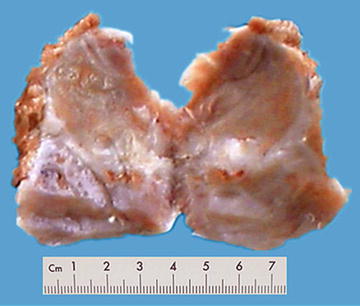

Fig. 6.49
Infantile hemangioendothelioma. The cross section was yellow white with unclear boundary
6.3.3.4 Microscopic Features
IHE can be divided into two pathological types:
Type I accounts for about 80% of the tumors. The surrounding structure was composed of dense proliferation of irregular thin-walled capillary cavities, lined with monolayer of fat or flat endothelial cells (Figs. 6.50 and 6.51), which are consistent in morphology and contain small- or medium-sized nuclei and eosinophilic nucleoli, with little nuclear fission. The cavities contain red blood cells, and fewer tumorous interstitial components can be found with visible residue of hepatocytes, bile duct, extramedullary hematopoiesis regions, and no fibrous envelope. The infiltration border was interspersed in the liver between the plates (Fig. 6.52). Moreover, hemorrhage, necrosis, and calcification can also be found in the tumor. Type I IHE can undergo canceration.