Introduction
Atherosclerotic cardiovascular disease (ASCVD) is a major cause of morbidity and mortality among adults in industrialized countries. Dyslipidemia (specifically elevated low-density lipoprotein [LDL] cholesterol, low high-density lipoprotein [HDL] cholesterol, and high non-HDL cholesterol and triglycerides [TGs]) has been identified as an independent risk factor in the development of ASCVD. There is strong evidence that lipoprotein levels track from childhood into adulthood and that abnormal levels of LDL cholesterol and perhaps other lipoproteins are associated with atherosclerosis, and therefore with related adverse outcomes.
This chapter reviews the evidence for the role of lipid abnormalities in the early natural history of atherosclerosis. In addition, a general overview of lipoprotein metabolism is provided—followed by a review of genetic disorders in the metabolism of lipoproteins. Secondary causes of high cholesterol are explained, including the increasing prevalence of obesity and metabolic syndrome, as a cause of lipid abnormalities in the pediatric population. Standards and approaches to screening for hyperlipidemia in children are reviewed, as well as current approaches to the dietary and pharmacologic management of pediatric lipid disorders.
Metabolism
Lipid disorders in children and adolescents can result from defects in the production, transport, or degradation of lipoproteins. To understand the diverse causes of lipoprotein abnormalities, a brief review of lipoprotein structure, function, and metabolism is provided. Table 25.1 summarizes the lipoprotein subclasses, the source of each one, and the constituent lipids and apolipoproteins associated with each particle.
| Lipoprotein | Apolipoprotein | Source | Lipid Constituents |
|---|---|---|---|
| Chylomicrons | ApoB-48, apoC-II, a apoC-III, apoE a | Intestine | Dietary triglycerides |
| VLDL | ApoB-100, C-II, a C-III, a apoE a | Liver | Endogenous cholesterol and triglyceride |
| IDL | ApoB-100, apoE | VLDL metabolism | Cholesterol and triglyceride |
| LDL | ApoB-100 | VLDL metabolism | Cholesterol |
| HDL | ApoA-I, apoA-II, apoC-II, apoE | Liver and intestine | Cholesterol and phospholipid |
TGs, cholesterol esters, phospholipids, and plant sterols within food postingestion are digested to fatty acids, 2-monoglycerides, lysophospholipids, unesterified cholesterol, and plant sterols. Absorption of these digestive end products occurs through two mechanisms: passive diffusion and carrier-mediated transport. In passive diffusion, nonpolar lipids are solubilized with the aid of bile acids and lysophospholipids into mixed micelles that can diffuse through the apical surface of the enteric membrane. Carrier-mediated transport involves several different transport proteins for fatty acids and sterols. CD 36/scavenger receptor B2 (SR-B2), a fatty acid translocase, promotes long-chain fatty acid and cholesterol absorption in the proximal small intestine. At least two additional transporters, Niemann-Pick C1-like 1 protein (NPC1L1) and SR-B1, play a role in sterol uptake. As such, NPC1L1 and SR-B1 are targets for the cholesterol-lowering medication ezetimibe, a potent inhibitor of cholesterol and plant sterol absorption.
Most of the plant sterols ingested and about half of the absorbed cholesterol are excreted from the intestinal cell back into the lumen by two adenosine triphosphate (ATP)-binding cassette (ABC) half-transporters, G5 and G8, thus limiting the amount of sterols that are absorbed. A rare mutation of either ABCG5 or ABCG8, known as sitosterolemia , results in abnormally high plant sterol levels in plasma and tissues and deposition of sterols in the skin and arteries. Individuals with this disorder are at an increased risk of premature atherosclerosis. Sterols that remain in the enterocyte are converted to sterol esters by acyl-CoA cholesterol acyl transferase (ACAT), which attaches a fatty acid to the sterol for storage within the cytoplasm of the cell. Within the enterocyte, lipids are aggregated into lipoproteins through the action of a chaperone protein, microsomal triglyceride transfer protein (MTTP), and perhaps several additional proteins. MTTP conjugates TGs, phospholipids, cholesterol, and cholesterol ester with apolipoprotein B-48 (apoB-48) on the luminal side of the endoplasmic reticulum (ER) membrane to create a mature chylomicron. A similar process is used to aggregate TG, phospholipids, cholesterol, and cholesterol ester with apoB-100 in the liver to form very low-density lipoprotein (VLDL) particles. In the genetic disorder abetalipoproteinemia, mutations in the gene encoding MTTP result in an inability to produce chylomicrons and VLDL, suggesting the essential nature of MTTP in chylomicron and VLDL biogenesis. The recently approved drug lomitapide inhibits MTTP, reducing lipoprotein assembly and secretion, and lowers plasma cholesterol by around 50% in patients with homozygous familial hypercholesterolemia.
Chylomicrons once formed are too large to penetrate the capillary membrane. Consequently, they are secreted into the lymphatic system and enter the venous plasma compartment through the thoracic lymph duct. As the nascent particles are released into the plasma, several apolipoproteins (including apoC-II, C-III, and apoE) are preferentially transferred to the chylomicrons from circulating HDLs. Fig. 25.1 depicts chylomicron metabolism.
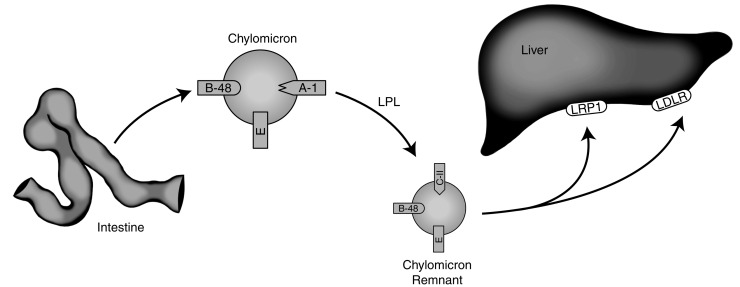
Chylomicrons transport dietary TG and cholesterol to sites of storage or metabolism. The size of the particles varies depending on the amount of fat ingested. They are rapidly cleared from the circulation through the action of lipoprotein lipase (LPL). LPL is a TG hydrolase found on the capillary endothelium of various tissues, with its highest concentration in muscle and adipose tissues. Glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 (GPIHBP1) anchors LPL to the capillary endothelium. LPL is activated by apoC-II and inhibited by apoC-III on the chylomicron. Loss-of-function mutations in LPL, apoC-II, and GPIHPB1 can result in marked hypertriglyceridemia. Loss-of-function mutations in apoC-III are associated with increased LPL activity and decreased TG levels. As the TG contained within the chylomicron is hydrolyzed, free fatty acids are liberated for oxidation via a variety of cell types and the particle decreases in size. When approximately 80% of the initial TG has been removed, apoC-II dissociates from its surface. The transfer of apoC-II from chylomicrons to HDL decreases the ability of LPL to further breakdown TGs. The TG-depleted chylomicrons, now considered chylomicron remnants, are taken up by the liver through the LDL receptor (LDLR), a receptor that recognizes apoE on the chylomicron surface and apoB100 on the surface of liver-derived lipoproteins. A smaller fraction of remnants may also be internalized via an LDLR-related protein-1 (LRP1)-mediated endocytosis.
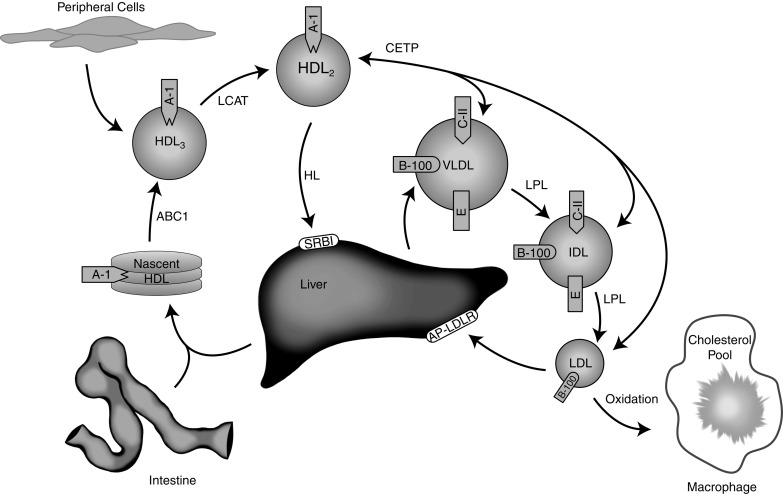
VLDLs originate from the liver, and like chylomicrons they are TG-rich particles ( Fig. 25.2 ). In contrast to the intestinally derived chylomicrons, the fatty acids contained within the VLDL TG come from de novo synthesis from dietary carbohydrate, lipoprotein remnants, or circulating fatty acids, internalized by the liver from plasma. Similar to chylomicrons, the size of the VLDL particles can vary depending on the quantity of the TG carried in the particle. When TG production in the liver is increased, the secreted VLDL particles are large. Within the hepatocyte, TG and cholesterol ester are assembled by an MTTP and surrounded with a phospholipid membrane associated with apoB-100. The mature VLDL particles are released into the lymph and ultimately into the vascular space, where other apolipoproteins (including apoC-II, apoC-III, and apoE) adsorb to the VLDL surface. The metabolism of the VLDL particle follows a route similar to that of the chylomicron: apoC-II on its surface activates LPL, LPL hydrolyzes the VLDL TG, free fatty acids are liberated, the particle decreases in size (after an 80% loss of TG), and ultimately apoC-II dissociates—resulting in the formation of VLDL remnants (also known as intermediate-density lipoproteins [ IDLs ]). Approximately half of the IDL is then removed from plasma through the interaction of apoE with the LDLR and LRP1 on the surface of liver cells. The rest of the IDL is converted to LDL through further hydrolysis of TGs and phospholipids by hepatic TG lipase (HL). ApoE is transferred from IDL to HDL during the transition of the remnant to LDL.
LDL, the major carrier of cholesterol in plasma, is taken up into peripheral tissues and liver cells by the LDLR assisted by an adaptor protein (AP). The AP binds to the LDLR and clathrin, suggesting a role for AP in the recruitment and retention of LDLR in clathrin-coated pits. Upon receptor binding, the LDL particle bound to LDLR/AP is rapidly internalized into clathrin-coated pits by endocytosis. Within the cell, the newly formed endosome becomes acidified through the action of an ATP-dependent proton pump. Acidification causes degradation of the clathrin coat, dissociation of the LDLR from LDL, and subdivision of the endosomal membranes. The endosome containing the LDLR recirculates back to the cell membrane for additional LDL uptake. Alternatively, proprotein convertase subtilisin/kexin type 9 (PCSK9) binds LDLR, and short-circuits recycling of LDLR from the endosome, leading to its degradation. The remaining LDL-containing endosome fuses with a lysosome, where hydrolytic enzymes digest the lipoprotein into its component parts: unesterified cholesterol, fatty acids, and free amino acids.
The amount of cholesterol released from endosomal uptake regulates hepatic synthesis of LDLR and cholesterol. When cellular concentration of cholesterol is low, sterol receptor binding proteins (SREBPs) move from the ER to the Golgi, where proteases cleave SREBPs into active transcription factors. SREBPs translocate to the nucleus, where they stimulate the transcription of LDLR and hydroxymethylglutaryl (HMG) CoA reductase, the rate-limiting enzyme of cholesterol biosynthesis. If cholesterol levels in the cell are high, SREBPs remains in the ER in an inactive form and do not stimulate LDLR synthesis. In this way, intracellular hepatic cholesterol concentration regulates the amount of cholesterol internalized and synthesized by the cell.
When excess LDL and other small apoB-containing lipoproteins (chylomicron remnants and IDL) are present in the plasma, the capacity of the LDLR to remove them is exceeded and these particles become more susceptible to oxidation. Oxidized apoB-containing lipoproteins can be taken up by scavenger receptors on macrophages in the subendothelium of arteries and may contribute to the formation of atherosclerotic lesions.
HDL transfers cholesterol and other lipids from peripheral tissues (including arterial atheroma) back to the liver. The particles are synthesized predominantly in the liver (and to a lesser extent in the intestine) as lipid-poor precursor particles (pre-beta HDL) containing apoA-I (see Fig. 25.2 ). Nascent HDL interacts with the plasma membrane of cells, collecting lipid through an ABCA1 mechanism. The cholesterol and phospholipids transferred through this process adsorb to the HDL, forming a disk-shaped particle referred to as HDL3 . Dysfunction of ABCA1 will significantly decrease HDL levels and thereby dramatically impair cholesterol and lipid transport functions. A rare autosomal recessive disorder called Tangier disease is caused by lack of functional ABCA1 protein and is characterized by an absence of HDL along with hypertriglyceridemia and low LDL levels. Within the plasma, HDL3 interacts with the enzyme lecithin cholesterol acyl transferase (LCAT)—which catalyzes the esterification of particle-associated cholesterol. ApoA-I on the HDL surface activates LCAT. Once formed, the cholesterol ester is more hydrophobic and moves to the interior of the particle—creating a sphere-shaped HDL particle known as HDL2 .
As HDL2 increases in size, the particle becomes substrate for cholesterol ester transfer protein (CETP). This enzyme promotes the exchange of esterified cholesterol within HDL2 for TG contained within apoB-100–associated lipoproteins. This lipid exchange is the primary mechanism whereby HDL participates in reverse cholesterol transport from tissues back to the liver. The rest of the cholesterol ester is selectively taken up from HDL by hepatocytes via a SR-B1, without concomitant uptake of the entire HDL particle. This latter process may require the action of HL. The lipid-poor pre-beta HDL resulting from this process is released for recycling.
Primary Dyslipidemias
Lipoprotein synthesis, transport, and metabolism occur in many steps and involve many specialized proteins. A number of genetic defects have been identified in these processes and are referred to as primary dyslipidemias . Most of these genetic defects present in childhood. Table 25.2 summarizes pediatric lipoprotein disorders with reference to the characteristic lipoprotein profile of each one. The genetic and metabolic etiologies of these disorders are detailed in the following material.
| Lipoprotein Disorder | Lipoprotein Analysis | Blood Lipids | Genetic Defect |
|---|---|---|---|
| Familial hypercholesterolemia | ↑↑LDL | ↑↑Cholesterol | LDL receptor ( LDLR ) |
| Autosomal recessive hypercholesterolemia | ↑↑LDL | ↑↑ Cholesterol | LDLRAP |
| Autosomal dominant hypercholesterolemia | ↑↑LDL (with increase in function mutations) | ↑↑ Cholesterol | PCSK9 |
| Familial ligand-defective apoB-100 | ↑↑ LDL | ↑↑Cholesterol | ApoB-100 |
| Sitosterolemia | ↑ LDL | ↑ Cholesterol | ABCG5 or ABCG8 |
| Familial combined hyperlipidemia | ↑ VLDL, ↑ LDL, ↓ HDL | ↑ Cholesterol, ↑ triglycerides | Unknown |
| Familial hypertriglyceridemia | ↑↑ VLDL, ↓ HDL | ↑ Triglycerides | Unknown |
| Familial chylomicronemia syndrome | ↑↑ Chylomicrons ↑ VLDL | ↑↑Triglycerides | Lipoprotein lipase ( LPL ), ApoC-II , Apo A-V , GP1HBP1 |
| Hypoalphalipoproteinemia | ↓ HDL | Normal | ApoA-1 |
| Dysbetalipoproteinemia | ↑↑ Chylomicron remnants, ↑↑ IDL | ↑↑ Cholesterol, ↑↑ triglycerides | ApoE |
Disorders of Cholesterol Metabolism
Familial Hypercholesterolemia
Familial hypercholesterolemia (FH) is the most common single gene disorder of lipoprotein metabolism. FH is inherited as an autosomal-dominant trait with relatively low prevalence in Western countries. The prevalence has been reported to be 10 times higher in certain populations with a presumed founder effect, such as the Lebanese, the French Canadians, and the South Afrikaners. The heterozygous form is found in one in 250 persons, and the homozygous form is found in one in 1 million persons. The disorder is caused by a mutation in the LDLR gene. More than 1200 mutations in this gene have been identified, including those that affect receptor synthesis, intracellular transport, ligand binding, internalization, and recycling. In the heterozygous form, inheritance of one defective LDLR gene results in plasma LDL cholesterol levels 2 to 3 times higher than normal. TG and HDL cholesterol levels are usually unaffected by FH-causing gene mutations, but may be altered by obesity and insulin resistance.
Individuals with heterozygous FH are at an increased risk of developing early-onset ASCVD, usually between the ages of 30 and 60 years. In the homozygous form, individuals inherit a mutant allele for FH from both parents, resulting in plasma LDL cholesterol concentrations that are 4 to 6 times higher than normal. A more severe phenotype is found in individuals with receptor-negative mutations (those with 5% residual LDL receptor activity) compared with those with receptor-defective mutations (5%–30% of normal LDL receptor activity). Because of the excessively high plasma cholesterol levels in individuals with homozygous FH, cholesterol deposits are common in the tendons (xanthomas) and eyelids (xanthelasmas)—generally by the age of 5 years. In the heterozygous form, xanthomas occur less frequently and generally not until one reaches older adulthood. Children with homozygous FH have early-onset atherosclerosis and often have myocardial infarction in the first decade of life, and death from ASCVD in the second decade.
Autosomal-Dominant and Autosomal-Recessive Hypercholesterolemia
Autosomal-dominant hypercholesterolemia (ADH) is another inherited disorder resulting in a phenotype that is expressed as marked elevations or low levels of LDL cholesterol. ADH is caused by mutations in a serine protease, PCSK9. This protein binds and favors degradation of the LDLR and thereby modulates the plasma levels of LDL cholesterol. Some of the naturally occurring PCSK9 mutations result in an increase in the function of the protein and cause hypercholesterolemia by increasing the degradation of LDLR, whereas other mutations result in a loss of function, and hence increase in LDRL abundance, and are associated with low LDL cholesterol. The latter mutations appear to confer protection from developing ASCVD.
Autosomal recessive hypercholesterolemia (ARH) is caused by mutations in the ARH gene, which encodes the adaptor protein required for normal LDLR-mediated endocytosis in hepatocytes. Several different mutations in this protein have been identified, all leading to a lack of or suboptimal internalization of the LDLR. Cholesterol levels in individuals with ARH are 5 to 6 times higher than normal. Children with this disorder are clinically similar to those with homozygous FH. However, their parents usually have normal lipoprotein profiles.
Familial Ligand-Defective ApoB-100
Familial ligand-defective apoB-100 (FDB) is a monogenic disorder that clinically resembles heterozygous FH. The disease is characterized by moderate to markedly high plasma LDL cholesterol levels, normal TGs, and tendon xanthomas. The disorder is caused by poor binding of the LDL particle to the LDLR, because of a mutation in apoB-100. Specifically two mutations, R3500Q and R3500W remain the most frequently identified mutations that cause FDB. Deficient LDLR binding results in a decreased clearance of LDL from plasma. The disorder is most common in individuals of European descent (one per 1000). Patients with FDB are at moderate to high risk of developing ASCVD.
Sitosterolemia
Sitosterolemia is a rare autosomal-recessive disease caused by a mutation in either of two genes ( ABCG5 or ABCG8 ) encoding the ABC half-transporters. These genes are expressed in enterocytes and hepatocytes. The ABC half-transporters limit the absorption of cholesterol and plant sterols (and possibly shellfish sterols) in the gut. They also promote biliary and fecal excretion of cholesterol and phytosterols. Defective proteins result in an abnormally high absorption of plant sterols (and, to a lesser extent, cholesterol) into the enterocyte, and decreased excretion of these sterols from the liver into the bile. Plasma cholesterol can be mildly, moderately, or markedly elevated, whereas plant sterol concentrations in the plasma are markedly increased. Patients with sitosterolemia develop premature ASCVD and xanthomas in childhood, and may develop aortic stenosis.
Disorders of Overproduction of Very Low-Density Lipoprotein
Familial combined hyperlipidemia (FCHL) is an autosomal-dominant disorder with a prevalence of 1% to 2% in Western populations. There is overlap in the lipid phenotype between FCHL and combined dyslipidemia (CD) of obesity, which likely has genetic underpinnings but is primarily influenced by lifestyle factors. CD is highly prevalent in youth, occurring in 30% to 60% of obese children and adolescents. Individuals with FCHL and CD generally share the same metabolic defect, which is overproduction of hepatic VLDL. Families with FCHL have multiple patterns of hyperlipidemia, including hypercholesterolemia, hypertriglyceridemia, and elevated apoB levels. A diagnosis of FCHL is based on the presence of increased levels of cholesterol, TG, or apoB in patients and their first-degree relatives. Veerkamp and colleagues have developed a nomogram to calculate the probability that a person is likely to be affected by FCHL. FCHL can manifest in childhood, but is usually not fully expressed until adulthood. Patients with FCHL and CD often have concurrent problems with insulin resistance, central obesity, and hypertension and are at an increased risk of premature ASCVD.
Syndromes with a similar phenotype to FCHL and CD are hyperapobeta-lipoproteinemia, LDL subclass pattern B, and the clustering of ASCVD risk factors known as metabolic syndrome in adults . Of the three, the latter syndrome is much more prevalent in children. Rates of metabolic syndrome are continuing to rise with the prevalence of obesity in the pediatric population. There appears to be a mechanistic link between central obesity, insulin resistance, and dyslipidemia—with central obesity generally preceding both glucose and lipid abnormalities. Currently, there is no agreed upon definition for metabolic syndrome in childhood.
Disorders of Marked Hypertriglyceridemia
Familial Hypertriglyceridemia
Familial hypertriglyceridemia (FHTG) follows an autosomal-dominant inheritance pattern expressed predominantly in adulthood, with a population prevalence of around 5% to 10%. The prevalence in children is increasing. Obesity is an important factor that can expedite the expression of FHTG, and patients often have concurrent glucose intolerance. The phenotype for FHTG is moderate to markedly high serum TGs (200–500 mg/dL range) and low to normal LDL and HDL cholesterol levels. The metabolic cause of the disorder is hepatic secretion of large TG-rich VLDL particles that are catabolized slowly. The fundamental genetic defect for FHTG has not been identified.
Familial Chylomicronemia Syndrome
Chylomicronemia syndrome is a compilation of rare monogenetic disorders that cause marked impairment of LPL activity. These disorders are phenotypically expressed as hypertriglyceridemia (usually TGs > 1000 mg/dL), because of diminished or absent hydrolysis of chylomicron and VLDL-associated TGs by LPL. The estimated prevalence is one in 500,000 to 1,000,000. Impairment of LPL activity may be related to LPL deficiency, apoC-II (cofactor for LPL) deficiency, or the more recently described apoA5 and GPIHBP1 loss-of-function mutations that result in poor hydrolysis of chylomicron and VLDL-associated TGs.
In homozygous chylomicronemia, fasting plasma has a viscous, creamy appearance because of the presence of large numbers of chylomicron particles. Risks for pancreatitis and hepatosplenomegaly are increased because of the markedly elevated serum TGs. In addition, eruptive xanthomas and neurologic symptoms may be apparent. Individuals heterozygous for the syndrome may have a mild to moderate elevation in plasma TGs that can range from 200 to 750 mg/dL. Environmental factors, such as weight gain, may exacerbate hypertriglyceridemia. Premature cardiovascular disease (CVD) is generally not a feature of chylomicronemia, but cases have been reported.
Hypolipidemias
Low High-Density Lipoprotein Cholesterol
In clinical practice, patients with low HDL cholesterol levels commonly have concurrent high TGs, with or without elevations in small dense LDL cholesterol. These patients are usually obese, and the mechanistic explanation for this dyslipidemic triad is VLDL overproduction. Less common are familial disorders of HDL, including familial hypoalphalipoproteinemia, mutations of the apoA-1 protein, Tangier disease, and LCAT deficiency. These disorders are characterized by a low HDL cholesterol level, with no other lipid abnormality. Familial hypoalphalipoproteinemia follows an autosomal-dominant inheritance pattern. ApoA-1 levels are also often low because of decreased production of HDL.
A number of mutations have been described in the apoA-1 gene and are associated with low HDL cholesterol and low apoA-1. Tangier disease is caused by mutations in the ABCA1 gene. Patients affected by this disease are not able to actively withdraw cholesterol from cells onto nascent HDL particles, causing rapid degradation of the nascent HDL. ApoA-1 is rapidly cleared before it is able to acquire cholesterol. In Tangier disease, HDL cholesterol levels are close to zero and the apoA-1 levels are less than 5 mg/dL. The risk of premature ASCVD in these patients is mild to moderate. LCAT deficiency is a very rare autosomal recessive disorder caused by mutations in LCAT, an enzyme synthesized by the liver and secreted into the plasma, where it associates with lipoproteins. LCAT esterifies free cholesterol on the surface of HDL and enables the accumulation of cholesteryl esters in the core of HDL. In LCAT deficiency, lack of normal cholesterol esterification impairs formation of mature HDL particles, which are readily catabolized along with apoA-1. Remarkably, despite the extremely low levels of plasma HDL cholesterol (usually < 10 mg/dL) and apoA-1, premature CVD is not a consistent feature of this disorder.
Abetalipoproteinemia
Abetalipoproteinemia is associated with low serum cholesterol (< 50 mg/dL) and TGs (~ 2–45 mg/dL). Patients with this disorder present with steatorrhea and fatty liver. Without treatment, ataxia follows (with acanthocytosis and retinitis pigmentosa). Abetalipoproteinemia is caused by a defect in MTTP. Without MTTP, no chylomicrons, VLDL, or LDL appear in the plasma. In these patients, HDL takes over as the primary cholesterol carrier. Thus the defect is not fatal because of significant fat malabsorption, fat-soluble vitamin status is impaired.
In particular, because vitamin E absorption and cellular uptake require chylomicron and LDL transport, high doses of vitamin E are required to prevent retinal and sensory neuron degeneration. Additional dietary considerations include restricting long-chain dietary TGs to less than 15 g/day to alleviate the steatorrhea. Medium-chain triglycerides (MCT oils) can be used as an alternative source of energy.
Hypobetalipoproteinemia
Hypobetalipoproteinemia is an autosomal-dominant disorder resulting from a defect in the apoB gene that produces a truncated apolipoprotein B. Cholesterol levels in patients with heterozygous hypobetalipoproteinemia are usually 50% of those of an unaffected family member. The heterozygous form of this condition is benign. However, homozygous hypobetalipoproteinemia is associated with severe hypocholesterolemia, significant steatorrhea, fatty liver, acanthocytosis retinopathy, and peripheral neuropathy.
Loss-of-Function Mutations for PCSK9
PCSK9 loss-of-function mutations are associated with very low LDL-cholesterol levels and reduced ASCVD risk (see section on Autosomal-Dominant and Autosomal-Recessive Hypercholesterolemia for mechanism for low LDL-cholesterol level). These mutations are found in approximately 2% of the population. Compared with noncarriers, heterozygous carriers have a 28% reduction in LDL-C levels and an 88% reduction in ASCVD risk, and may also protect carriers from myocardial infarction. Preventing PCSK9-mediated LDLR degradation with monoclonal antibodies is a novel strategy for LDL-cholesterol lowering, in patients with severe forms of hypercholesterolemia.
Disorders With Lipoprotein Clearance via ApoE Pathways
Dysbetalipoproteinemia is characterized by elevated cholesterol and TG levels. The disorder results from the presence of a polymorphism of the apoE allele ( apoE2 , rather than the more common apoE3 or less common apoE4 ). Metabolically, this defect results in a poor uptake of remnant particles and abnormal remnant catabolism because of the abnormal apoE . Increased remnants, VLDL, chylomicrons, and apoE are all present. Xanthomas may occur, and premature ASCVD has been reported. This lipoprotein disorder is rare in children and often presents in young adulthood.
Secondary causes
Secondary dyslipidemias can result from a variety of diseases and conditions ( Box 25.1 ). In the United States, the most prevalent cause of secondary dyslipidemia is overweight and obesity. The dyslipidemic triad (namely, elevated TGs and small dense LDL and low HDL cholesterol) is commonly associated with overweight (in particular, with central adiposity). In addition to dyslipidemia, insulin resistance and elevated blood pressure may be present. This cluster of abnormalities is known in adults as the metabolic syndrome . Empirical evidence in children also indicates that obesity during childhood is associated with the same risk factor clustering seen in adults, that it continues into adult life, and that it is associated with an increased risk for accelerated early atherosclerosis. The primary approach to treating this disorder in both adults and children is weight management. Improvement in weight status and a decrease in body fatness have been shown to be associated with improvements in the dyslipidemia and other comorbidities associated with obesity.
Metabolic lipid perturbations in adult patients with types 1 and 2 diabetes mellitus are similar to those found in patients with the metabolic syndrome, but often are more severe. In general, in adults with diabetes, TGs are elevated and HDL cholesterol is low—and LDL cholesterol can be normal, mildly, or moderately elevated. Diabetes in adults is considered an ASCVD risk equivalent according to the National Cholesterol Education Program (NCEP). This means that the risk for developing ASCVD in patients with poorly controlled diabetes is equivalent to those with established ASCVD. For this reason, the NCEP recommends aggressive treatment of dyslipidemia in adult patients with diabetes.
Although type 1 diabetes is currently the main form of diabetes seen in children, in the United States a growing number of patients with type 2 diabetes are under the age of 18 years. Change in the prevalence of type 2 diabetes in youth is likely related to the growing obesity epidemic occurring in the pediatric population. Data on lipid concentrations in children and adolescents with diabetes are few, particularly in those with type 2 diabetes.
The Search for Diabetes in Youth Study assessed the prevalence of serum lipid abnormalities among a representative sample of US children and adolescents with type 1 and type 2 diabetes. Findings from this study showed a substantial number of diabetic children over the age of 10 years with abnormal serum lipids: nearly 50% had an LDL cholesterol level above the optimal level of 100 mg/dL. For children with type 2 diabetes, 37% had elevated TG levels and 44% had low HDL cholesterol. These data highlight the importance of serum lipid screening in children with diabetes. A growing body of literature also shows early vascular dysfunction in children with diabetes, regardless of type. This is thought to be caused by glycemic and lipid abnormalities associated with poorly managed diabetes. For this reason, new treatment guidelines recommend intensive glucose and lipid management for children with diabetes. These guidelines are discussed later in the chapter.
Other causes of secondary dyslipidemia include hypothyroidism, nephrotic syndrome, other renal diseases, liver diseases, and infection. The risk of development of atherosclerosis with these conditions is unknown but is likely proportionate to the length of exposure and extent of elevation in serum LDL cholesterol levels. CVD is common in patients with chronic renal insufficiency. Dyslipidemias can also result from the ingestion of a variety of medications. These medications include progestins, estrogens, androgens, anabolic steroids, corticosteroids, cyclosporine, and retinoids. Secondary causes of dyslipidemias should be identified by patient historical data and a careful physical examination. Laboratory tests (including thyroid, renal, and liver function panels) can confirm the diagnosis.
The treatment of dyslipidemia in patients with secondary causes is focused on managing the underlying disease. Diet and physical activity changes may also be recommended to reduce elevated LDL cholesterol and TG levels.
Vascular changes and dyslipidemia
It is well established that elevated concentrations of total cholesterol and LDL cholesterol in adult life are strong and reversible risk factors for ASCVD. Whether dyslipidemia during childhood contributes to atherosclerotic lesions in coronary and other arteries has been a subject of debate, but accumulating evidence from pathology and in vivo imaging studies favors a relationship. Atherosclerotic lesions result from deposits of lipid and cholesterol in the intima of the arterial wall. Early lesions, called fatty streaks , are formed from the accumulation of macrophages filled with lipid droplets (foam cells).
Fatty streaks do not disorganize the normal structure of the intima, do not deform or obstruct the artery, and are in and of themselves not considered harmful. However, some continue to accumulate macrophage foam cells and extracellular lipid and smooth muscle cells—forming raised plaques. From these, more advanced lesions may develop—with further deposition of extracellular lipid, cholesterol crystals, collagen, and potentially calcium. It is these raised lesions that result in a myocardial infarction because of their increasing size and obstruction of the arterial lumen or because of rupture of the fibrous plaque, which results in the release of thrombogenic substances from the necrotic core.
Pathobiological studies of the coronary arteries of young individuals who died from causes unrelated to heart disease have been useful in documenting the progression of atherosclerosis by age and risk factor determinants. Stary and colleagues studied more than 500 postmortem samples of coronary arteries from persons younger than 30 years of age and found the presence of fatty streaks in the majority of children younger than 9 years of age, raised lesions in about half of adolescents, and more advanced lesions in about one-third of the young adults studied. In 93 autopsies of young adults for whom childhood risk factor data were available, Berenson and colleagues found that the extent of the surface of arteries covered with fatty streaks and fibrous plaques was positively associated with LDL cholesterol, TGs, blood pressure, and body mass index (BMI), and negatively associated with HDL cholesterol levels in childhood.
The Pathobiological Determinants of Atherosclerosis in Youth study reached similar conclusions from examination of more than 3000 postmortem samples of coronary arteries of young adults who died from noncardiovascular events and who likewise had a variety of surrogates for antimortem risk factor measures available. In general, pathology studies have made important contributions to the identification of risk factors for early aspects of the atherosclerotic process. In conjunction with findings from longitudinal studies, such as the Framingham Heart Study (in which risk factor assessments of participants preceded the development of CVD), a group of risk factors, often referred to as the traditional risk factors for ASCVD has been established. A complete list of pediatric risk factors for ASCVD is found in Box 25.2 .
Positive risk factors
- •
Elevated LDL cholesterol (≥ 130 mg/dL)
- •
Family history of premature (aged > 55 years) coronary heart disease, CVD, or peripheral vascular disease
- •
Smoking
- •
Hypertension
- •
Obesity (≥ 95th percentile weight for height on National Center for Health Statistics [NCHS] growth chart)
- •
Physical inactivity
- •
Diabetes
Negative risk factors
- •
High HDL cholesterol (60 mg/dL)
Advances in vascular imaging technology have provided a means of measuring early pathologic changes and functional abnormalities against coronary and other arteries in response to adverse changes in CVD risk factors. The advantage in using this technology is that walls of superficial arteries can be imaged noninvasively in real time at high resolution, and changes to the arterial wall can be measured as a continuous variable from childhood to adulthood in patients with and without the presence of risk factors for ASCVD. Computed tomography (CT) scanning is considered one of the most sensitive noninvasive tools for imaging the extent and location of coronary artery calcium present in atheroma.
The presence of coronary artery calcium has been associated with adverse CVD outcomes in adults. In adolescents, several prospective studies have shown associations between risk factors for ASCVD in youth and coronary artery calcium in young adulthood. In the Muscatine Study, in which participants were assessed for CVD risk factors during their school-age years, and later assessed for cardiovascular changes by CT scan, 31% of men and 10% of women aged 29 to 37 years had significant coronary artery calcification. In this study, childhood risk factors associated with calcification were obesity, increased blood pressure, and low HDL cholesterol. In the Cardiovascular Risk in Young Finns Study, elevated total and LDL cholesterol, Apo-B levels and systolic blood pressure measured in adolescence were associated with coronary artery calcium in middle-age, highlighting the role of lifelong risk factor exposure to the pathophysiology of CVD. Gidding and colleagues showed significant coronary calcium by electron beam CT in 7 of 29 young adults with heterozygous familial hypercholesterolemia. Overweight was found to increase the likelihood of calcium being present in individuals already at high risk.
Vascular ultrasound imaging has been used to assess alterations in brachial artery flow-mediated dilation, which is a measure of endothelial function, and carotid intima-media thickness (IMT). In adults, both measures have been associated with adverse changes in traditional ASCVD risk factors, respond to normalization of risk factors, and are considered important early markers for the progression of atherosclerotic disease. Although fewer studies have used ultrasound technology to evaluate coronary arteries in the young, children with hypercholesterolemia have been assessed using measures of the carotid and brachial arteries and have been found to have abnormalities of carotid IMT and brachial artery vasodilation. Early initiation and longer duration of statin therapy in youth with FH was related to better carotid IMT at 10 years of follow-up, as demonstrated by no difference in rate of increased carotid IMT between youths with FH compared with their nonaffected siblings. Regression of carotid IMT has also been demonstrated in youth with treatment of dyslipidemia, whereas a study of statin therapy in low-risk adults demonstrated lack of progression of carotid IMT but did not result in regression of atherosclerosis. In a systematic review of 51 studies evaluating over 4000 FH patients and 700 FCHL patients, impaired endothelial function, as measured by reduced brachial artery flow-mediated dilation (FMD), was significantly improved with statins. Consistent with studies of carotid IMT, improvement was proportional to duration and intensity of statin therapy.
In summary, these studies confirm the utility of vascular imaging for detecting early pathologic and functional changes to coronary vessels, and associations with modifiable CVD risk factors in the young. Clinically, vascular imaging by ultrasound may be a valuable means of estimating the benefit of treating multiple CVD risk factors in children and adolescents. However, the collection of more normative data across age, race, and gender groups, and longitudinal studies to determine age and puberty-related changes in these measures is needed before these methods could be adopted in clinical evaluation. In general, CT scans may be less useful in younger patients because calcium depositions are uncommon before young adulthood.
Screening for lipid disorders
Routine Screening
The approach to pediatric screening for dyslipidemia has been controversial. Since the 1990s, pediatric guidelines established by the NCEP have provided the standard of care, with respect to lipid screening and treatment of dyslipidemia in children. These guidelines recommend selective blood cholesterol screening in children, based on a positive family history of premature CVD (before age 55 years), presence of dyslipidemia in a parent (total cholesterol > 240 mg/dL), or presence of additional CVD risk factors in the child, such as hypertension, diabetes, and obesity. If family history is unknown, recommendations suggest that lipid screening of a child be done at the discretion of the primary care provider. Selective screening is endorsed for young children between 2 to < 9 years of age by the National Heart, Lung and Blood Institutes (NHLBI) Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents.
For older children ages 9 to 11 years and again after puberty (ages 17–21 years), the NHLBI Expert Panel recommends universal screening for dyslipidemia. This is based on evidence that targeted screening for dyslipidemia reportedly missed many children with moderate dyslipidemia (as many as 30%–60%) and failed to detect a substantial number who likely had genetic dyslipidemia who might require more intensive therapy. FH is a relatively common problem, with the heterozygous form occurring in one in 250 individuals. FH has been clearly associated with an increased lifetime risk of ASCVD, and earlier treatment is associated with reduced subclinical evidence of atherosclerosis. To increase the likelihood of detecting young patients with FH and other genetic dyslipidemias, the American Heart Association (AHA) and the National Lipid Association also endorse lipid screening during childhood and adolescence. The US Preventive Services Task Force 2016 guidelines on pediatric lipid screening do not specifically advocate for or against pediatric lipid screening, but cite a lack of knowledge regarding the relationship between childhood lipid levels and hard cardiovascular outcomes, and the need for further evidence in this regard.
The NHLBI Expert Panel recommendations recognized that there is considerable variation in LDL cholesterol with age during growth and development, especially during puberty. Total and LDL cholesterol levels tend to decline during puberty, meaning that some adolescents will appear normal, when in fact they will have elevated levels after puberty. For this reason, 9 to 11 years of age was selected as a good age to screen, before the effect of puberty lowers LDL cholesterol levels, but closer to an age when drug therapy may be appropriate.
The Expert Panel also included non-HDL cholesterol as a screening tool for the identification of dyslipidemia in children. Non-HDL cholesterol is calculated by subtracting the HDL cholesterol from the total cholesterol. This measure reflects the amount of cholesterol carried by atherogenetic apolipoprotein B–containing lipoproteins (VLDL, IDL, and LDL). In both adults and children, non-HDL cholesterol has been determined to be more predictive of persistent dyslipidemia and therefore atherosclerosis and future CVD events than total cholesterol, LDL cholesterol, or HDL cholesterol alone. A major advantage of non-HDL cholesterol is that it can be accurately calculated in a nonfasting state and is therefore practical to obtain in a primary care setting. Percentiles for non-HDL cholesterol have been established ( Table 25.3 ) and are based on data from the Bogalusa Heart Study, where non-HDL cholesterol greater than or equal to the 95th percentile is considered “abnormal/high” and between the 75th to 95th percentile is considered “borderline.”