Fig. 17.1
CNS tumor all-cause and sex-specific mortality rate compared with age-adjusted US population (Armstrong et al. 2009a) (Reprinted with permission ©2009 Oxford University Press, all rights reserved)
17.2.2 Secondary Malignancy
Chemotherapy-associated hematopoietic second malignancies typically occur within the first decade after treatment of the primary malignancy. Solid tumor secondary malignancies are usually radiation related and occur late, even several decades after initial therapy. In a study using CCSS data to assess secondary malignancies in all pediatric cancer survivors, the cumulative incidence of secondary malignant neoplasms was 7.9 % at 30 years from diagnosis (Friedman et al. 2010). Among survivors of pediatric CNS tumors, the incidence of either a benign or malignant secondary neoplasm was 10.7 % at 25 years from diagnosis (Armstrong 2010). Notably, there is no plateau of risk over time. Radiation is a major risk factor for the development of secondary malignancy, with the cumulative incidence of a subsequent CNS neoplasm of 7.1 % at 25-year follow-up in patients who received radiation versus 1.0 % in those who did not (Armstrong et al. 2009a). Additionally, multiple studies have identified a dose-response relationship between radiation dose received and the development of subsequent neoplasms (Armstrong 2010). The delayed nature of secondary malignant neoplasms associated with radiation therapy may lead to early underestimation of risk (Schiff and Wen 2006). The most common radiation-associated brain tumors are meningiomas, followed by gliomas and sarcomas (Behin and Delattre 2002). The CNS secondary malignancies occurred in survivors of other malignancies more often than in survivors of a CNS primary cancer. In all survivors of childhood cancer, the significant risk factors for a secondary malignant neoplasm adjusted for therapeutic radiation exposure included female sex and young age at diagnosis.
17.2.3 General Late Effects
In addition to the increased risk of secondary cancer and death, survivors of childhood CNS malignancies are at risk for morbidity related to injury from the tumor itself and long-term effects of radiation, surgery, and chemotherapy (Butler et al. 1994; Packer and Mehta 2002; Nathan et al. 2007; Turner et al. 2009). The CNS tumor survivors have among the highest morbidity rates of all pediatric cancer survivors (Hays et al. 1992; Foreman et al. 1999).
Pediatric cancer survivors treated with radiotherapy alone (55 %) have an increased risk of late adverse events when compared to patients treated with chemotherapy (15 %) or surgery (25 %) alone (Geenen et al. 2007). The CNS injury from radiation and chemotherapy is primarily due to cortical and subcortical white-matter changes, including glial-cell damage, and demyelination (Nathan et al. 2007). Radiation injury to CNS microvasculature results in hypoxia, as well as impairment of normal neurogenesis and synaptic plasticity (Tofilon and Fike 2000; Schiff and Wen 2006). Survivors of pediatric CNS tumors who received radiation therapy show decreased white-matter volume compared to those who did not (Reddick et al. 2000; Rueckriegel et al. 2010).
Oeffinger used the CCSS database to assess chronic health conditions in pediatric cancer survivors. His analysis found that survivors were 3.3 times as likely as their siblings to have a chronic health condition. The CNS tumor survivors were among the survivors at highest risk for a grade 3 or 4 chronic health conditions using the Common Terminology Criteria for Adverse Events, Version 3 (Fig. 17.2). Female survivors of any cancer were found to be at greater risk for any, a grade 3 or higher, and multiple chronic medical conditions (Oeffinger et al. 2006). For all diagnoses, the cumulative incidence of chronic health conditions continued to slowly increase over time, even beyond the fourth decade of life (Armstrong et al. 2014).
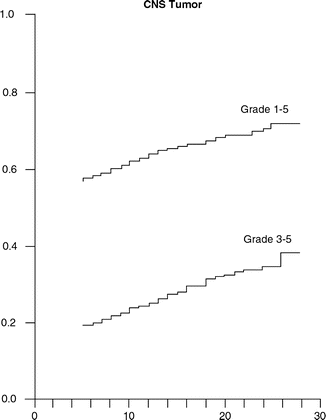

Fig. 17.2
In 1,322 adult survivors of pediatric CNS tumors, the incidence of chronic health conditions continues to increase over time (Oeffinger et al. 2006) (Copyright 2006 Massachusetts Medical Society. All rights reserved)
The CCSS examined general health outcomes among 9,535 childhood cancer survivors. The CNS tumor survivors reported increased adverse outcomes in general health, functional status, and activity status and were twice as likely to have at least one negatively affected domain when compared to other pediatric cancer survivors (Hudson et al. 2003). The most commonly affected medical domains for CNS tumor survivors include neurologic, neurocognitive, neuropsychological, endocrine, and other organ dysfunctions (Table 17.1).
Table 17.1
Chronic health conditions in pediatric CNS tumor survivors
Organ system | Chronic health condition |
|---|---|
Neurological | Paralysis |
Seizure | |
Fatigue | |
Chronic pain | |
Spasticity | |
Ataxia | |
Dysarthria | |
Ocular | Diplopia |
Cataracts | |
Visual loss | |
Dry eyes | |
Auditory | Tinnitus |
Hearing loss | |
Neurocognitive | Learning deficits |
Executive function (planning and organization) | |
Sustained attention | |
Memory | |
Processing speed | |
Visual-motor integration | |
Diminished IQ | |
Behavioral change | |
Neuropsychiatric | Social withdrawal |
Depression | |
Anxiety | |
Posttraumatic stress | |
Endocrine | Gonadal dysfunction, gonadotropin deficiency, infertility |
Metabolic syndromes, obesity | |
Growth hormone deficiency | |
Precious puberty | |
Hyperprolactinemia | |
Central hypothyroidism | |
Central adrenal insufficiency | |
Pulmonary | Pulmonary fibrosis |
Interstitial pneumonitis | |
Restrictive lung disease | |
Obstructive lung disease | |
Gastrointestinal | Dysphagia |
Esophageal stricture | |
Bowel obstruction | |
Chronic enterocolitis | |
Fistula | |
Strictures | |
Hepatic dysfunction | |
Cardiovascular | Congestive heart failure |
Cardiomyopathy | |
Pericarditis | |
Pericardial fibrosis | |
Valvular disease | |
Myocardial infarction | |
Arrhythmia | |
Atherosclerotic heart disease | |
Stroke | |
Vasculopathy | |
Renal | Impaired function |
Musculoskeletal | Osteopenia |
Osteonecrosis | |
Dermatologic | Alopecia |
Scarring | |
Dental | Tooth/root agenesis |
Root thinning/shortening | |
Enamel dysplasia | |
Dental caries |
17.2.4 Neurologic
Multiple studies have analyzed questionnaire data from CCSS primary CNS tumor patients to assess long-term neurologic and neurosensory deficits (Packer et al. 2003; Armstrong et al. 2009a). When compared to siblings, survivors are at a significant increased risk for late onset of legal blindness, cataracts, and double vision. Twelve percent of patients report hearing impairment with a statistically significant relationship to posterior fossa irradiation greater than 50 Gy (Packer et al. 2003) and a dose-dependent increase in risk with doses above 32 Gy (Merchant et al. 2004). Children with primitive neuroectodermal tumors (PNET) are at higher risk for developing hearing deficits than other CNS tumor survivors. Focal neurologic dysfunction is common, with 49 % of patients reporting coordination problems and 26 % reporting a motor control problem. Seizure disorder is reported in 25 % of patients, and 6.5 % of this group reported the first seizure five or more years after initial diagnosis (Packer et al. 2003). Seizures, hand-eye coordination problems, and hemiplegia have been associated with supratentorial tumors, while ataxia and balance have been associated with infratentorial tumors (Lannering et al. 1990). Chronic progressive radiation myelopathy can occur following spinal radiation (Schiff and Wen 2006). Fatigue has been shown to be associated in pediatric cancer survivors with a poor health-related quality of life (Meeske et al. 2007) as well as poor neurocognitive function (Clanton et al. 2011). However, there are conflicting reports on the impact of cancer therapy on long-term fatigue (Zebrack and Chesler 2002; Langeveld et al. 2003; Mulrooney et al. 2008). In a study of 176 childhood cancer survivors, including 19 CNS tumor patients, all study enrollees reported ongoing fatigue on a quality-of-life questionnaire (Zebrack and Chesler 2002). Conflicting with this outcome, a report of 416 pediatric cancer survivors, which included 30 CNS tumor survivors, found no evidence of excess fatigue in brain tumor survivors or pediatric cancer survivors overall (Langeveld et al. 2003). Analysis of the CCSS cohort indicated significantly increased scores on standardized measures of fatigue among survivors of pediatric CNS tumors compared to the sibling group, though with questionable clinical significance (Mulrooney et al. 2008). Chronic pain has also been reported as a late effect in 19–33 % of pediatric brain tumor survivors in a series of 52 and 44 patients, respectively (Barr et al. 1999; Foreman et al. 1999), though in a study of the CCSS cohort, pediatric CNS tumor survivors had a lower risk of reporting pain conditions compared with the sibling group (Lu et al. 2011).
17.2.5 Neurocognitive
Neurocognitive dysfunction can be a debilitating consequence and a predominant late effect of cancer therapy. Between 40 % and 100 % of pediatric CNS tumor survivors report neurocognitive problems (Moleski 2000; Oeffinger et al. 2008). Severity and probability of neurocognitive deficits are related to age at diagnosis and treatment, female gender, dose and volume of radiation given, dose and type of chemotherapy, hydrocephalus, and tumor type, size, and location (Packer et al. 1989; Radcliffe et al. 1992; Ris et al. 2001; Oeffinger et al. 2008; Ellenberg et al. 2009; Duffner 2010). Socioeconomic status has also been identified as a risk factor in some studies (Nathan et al. 2007). It is essential to monitor at-risk patients over time as a nonlinear decline in intellectual function is often seen. Although earlier-learned information is typically retained, the ability to acquire new information at the same rate as one’s peers is impaired (Mabbott et al. 2005). Children who have received brain irradiation may have cognitive dysfunction years after treatment that is independent of the number of school days missed due to therapy (Oberfield et al. 1986; Radcliffe et al. 1992; Ris et al. 2001; Padovani et al. 2012).
The most common neurocognitive impairments are problems with attention and concentration, processing speed and visual perceptual skills, executive function, and memory (Mulhern et al. 1998; Moleski 2000; Oeffinger et al. 2008). Deficits in full-scale intelligence quotient, verbal intelligence quotient, performance intelligence quotient, nonverbal memory, and somatosensory functioning have also been reported. Intelligence quotient scores have been shown to decrease as much as 15–25 points from baseline (Nathan et al. 2007). This decline increases in severity with increasing time from treatment (Askins and Moore 2008). The CCSS reported that 18 % of 18–24-year-old brain tumor survivors had not completed high school. In addition, brain tumor survivors often required special education services, with use increasing based on younger age at diagnosis. About 70 % of brain tumor survivors diagnosed before the age of 6 required special education services in school, compared with 24 % in those diagnosed after age 15 (Mitby et al. 2003). Multiple studies have examined the role of pharmacological stimulants in improving neurocognitive function in pediatric CNS tumor survivors (Castellino et al. 2012; Smithson et al. 2013). A recent review identified four original trials that demonstrated improved attention, processing speed, and cognitive flexibility in pediatric CNS tumor survivors treated with methylphenidate (Smithson et al. 2013). Additionally, trials evaluating the effects of acetylcholinesterase inhibitor donepezil and dopaminergic CNS stimulant modafinil on neurocognitive function in childhood brain tumor survivors are ongoing.
17.2.6 Neuropsychology
Neuropsychological effects of brain tumor therapy include general behavioral problems, maladjustment, depressive symptoms, and poor self-concept (Carpentieri et al. 2003). Some more severe psychiatric complications include emotional dysfunction and psychosis. Seventeen percent of CCSS survivors have depressive, somatic, or anxious symptoms (Hudson et al. 2003), with a subset of these survivors reporting persistently elevated distress symptoms (Brinkman et al. 2013). Nine percent report functional impairment and/or clinical distress consistent with a diagnosis of post-traumatic stress disorder (Stuber et al. 2010). Specifically, cerebellar damage has been associated with neuropsychological and psychiatric problems (Steinlin et al. 2003). There is some evidence to suggest that survivors of CNS tumors are at increased risk of hospitalization for psychiatric disorders (Ross et al. 2003). Within the CCSS cohort, CNS tumor survivors reported the highest prevalence of suicidal ideation compared with other pediatric tumor survivors and controls (Recklitis et al. 2010). While previous analyses have not indicated an increased risk of suicide mortality in pediatric tumor survivors compared with the general population (Mertens et al. 2008; Armstrong et al. 2009b), suicidal ideation has been associated with increased all-cause mortality in survivors of childhood cancer (Brinkman et al. 2014).
17.2.7 Psychosocial
Using CCSS self-reported employment history, Pang and colleagues examined survivor employment status. Of the cancer survivors, 5.6 % had never been employed compared to 1.2 % of the sibling group. The CNS tumor survivors had the highest risk of having never been employed (odds ratio [OR] = 9.9), although all survivors were at increased risk (OR = 3.7). Within the group of CNS tumor survivors, risk for unemployment by treatment modality was also evaluated: surgery alone (OR = 3.7), radiotherapy and surgery (OR = 11.8), and chemotherapy, radiotherapy, and surgery (OR = 10.7) (Pang et al. 2008). Survivors are additionally employed in lower-skill jobs than siblings (Kirchhoff et al. 2011). Survivors of CNS tumors are less likely than siblings to be married, have an income of greater than $20,000, and graduate from college (Armstrong et al. 2009a). Though survivors of other pediatric tumors report high levels of current and predicted life satisfaction, brain tumor survivors predict lower levels of life satisfaction 5 years into the future compared with sibling controls (Zeltzer et al. 2008).
17.2.8 Endocrine
Endocrine dysfunction is common in CNS tumor survivors. Children with suprasellar brain tumors have a high incidence of hormonal dysfunction (Ogilvy-Stuart et al. 1991; Sklar and Constine 1995). Patients who have received high-dose irradiation to the hypothalamic region can develop delayed-onset hormonal deficiency (Oberfield et al. 1986; Ogilvy-Stuart et al. 1991; Sklar and Constine 1995). Specific endocrinopathies include hypothyroidism, growth hormone deficiency, precocious puberty and/or gonadotropin deficiency, adrenocorticotropic hormone deficiency, panhypopituitarism, and diabetes insipidus (Rutter and Rose 2007; Nandagopal et al. 2008). Overall, patients treated with surgery alone manifest much lower rates of endocrine abnormalities than patients who also received radiation and/or chemotherapy (Gurney et al. 2003a). Young age at diagnosis increases risk of hypothalamic-pituitary axis dysfunction (Gleeson and Shalet 2004) (Gurney et al. 2003b).
Growth hormone deficiency is the most common endocrinopathy in pediatric CNS tumor survivors (Muirhead et al. 2002; Gurney et al. 2003a; Nandagopal et al. 2008). Livesey’s series of 144 CNS tumor survivors showed laboratory evidence of growth hormone deficiency in 97 % of the survivors at a median follow-up time of 9.6 years (Livesey et al. 1990). Risk factors for growth hormone dysfunction include increased radiation dose, fewer radiation fractions, and younger age at diagnosis (Darzy and Shalet 2009). Cranial radiation doses as low as 18 Gy can affect the growth hormone axis (Brownstein et al. 2004). Growth hormone deficiency in children results in growth failure and short stature. Recent studies have also demonstrated that growth hormone deficiency can have effects on adults including abnormal body composition, reduced lean body mass, increased abdominal adiposity, reduced strength and exercise capacity, impaired psychological well-being, depressed mood, reduced vitality and energy, emotional lability, impaired self-control, anxiety, and increased social isolation (Carroll et al. 2000). Investigations have shown no increased risk for disease recurrence in CNS tumor survivors treated with growth hormone therapy (Moshang et al. 1996; Sklar et al. 2002). Initial reports assessing the CCSS cohort indicated a small increase in the overall risk for subsequent neoplasms in survivors treated with growth hormone (Sklar et al. 2002), though this risk diminished with longer duration of follow-up (Ergun-Longmire et al. 2006) and was not found when analysis was limited to CNS subsequent neoplasms (Patterson et al. 2014).
Central thyroid dysfunction in addition to growth hormone dysfunction can cause obesity syndrome due to the compounded effects on linear growth, in addition to the effects of hypothyroidism. These hormonal imbalances likely contribute to the increased incidence of obesity in female survivors of pediatric brain tumors. Abnormal thyroid function may also contribute to learning disabilities in this population (Anderson 2003). Gonadal dysfunction often occurs later than other endocrinopathies in pediatric CNS tumor survivors and may not be detected until puberty or early adulthood. Gonadal dysfunction includes a wide range of abnormalities from precocious or delayed puberty to infertility. Livesey found that following spinal radiation, 35 % of females had ovarian dysfunction, while only 3 % of males had testicular dysfunction (Livesey et al. 1990). The use of alkylating agents increases the risk of gonadal failure. The use of vinblastine, cytarabine, or cisplatin has also been associated with infertility (Thomson et al. 2002). Panhypopituitarism is usually only diagnosed in patients who have received greater than 40 Gy of cranial radiation. Central adrenal deficiency may present as failure to thrive, anorexia, dehydration, hypoglycemia, decreased weight gain, and hypotension. Hyperprolactinemia often manifests as galactorrhea and menstrual abnormalities.
17.2.9 Cardiovascular and Cerebrovascular
The vascular system can be affected by CNS tumor treatments as the result of damage to both the CNS vasculature and the heart itself. In an evaluation of 1,607 CNS tumor survivors, 18 % of patients reported problems, including primary arrhythmia, stroke, blood clots, and angina-like symptoms (Gurney et al. 2003a). Spinal irradiation may contribute to cardiac injury (Jakacki et al. 1993). In the CCSS cohort, pediatric brain tumor survivors were 6.1 times more likely to experience myocardial infarction than sibling controls (Mulrooney et al. 2009). Metabolic dysfunction as a result of treatment-related endocrinopathies and limitations on physical activity may further exacerbate the risk of cardiovascular disease. Stroke is an important late effect in childhood CNS tumor survivors and may contribute to neurologic and neurocognitive disability. In the CCSS cohort, pediatric CNS tumor survivors demonstrated an increased risk of stroke as compared with sibling controls, by as much as a 30-fold (Bowers et al. 2006; Mueller et al. 2013). The underlying pathology is thought to be a radiation-induced vasculopathy, with cranial radiation increasing risk of stroke in a dose-dependent manner. Other common cranial vasculopathies following cranial radiation in children include moyamoya disease, cavernous malformations, and cerebral microhemorrhages (Ullrich et al. 2007; Lupo et al. 2012; Gastelum et al. 2014).
17.2.10 Other
Pediatric brain tumor survivors in the CCSS cohort demonstrated increased rates of pulmonary fibrosis, chest wall abnormalities, chronic cough, and need for supplemental oxygen as compared with sibling controls (Huang et al. 2014). Pulmonary disease following treatment is often linked to the use of nitrosoureas in this patient population. Cranial radiation is associated with low bone mineral density in brain tumor survivors. Methotrexate and steroids used in treatment may also contribute to this problem (Nandagopal et al. 2008).
17.2.11 Patient Factors
17.2.11.1 Age
Children less than 3 years of age at the time of therapy are thought to be at greatest risk for late effects due to their immature stage of brain development. These patients almost universally require special education services and are unlikely to live independently as adults (Nathan et al. 2007). Many CNS tumor treatment protocols have attempted to postpone and reduce cranial radiation as well as explore more targeted radiotherapy methodologies in young children, with a potential reduction in late effects (Sands et al. 2010; Saha et al. 2014).
17.2.11.2 Site
There is controversy over the role that tumor location plays in outcome (Mulhern et al. 1992; Ater et al. 1996; Steinlin et al. 2003). Multiple studies have demonstrated that supratentorial tumors confer worse morbidity than infratentorial tumors (Ellenberg et al. 1987; Lannering et al. 1990). Tumors in the cerebral hemispheres can cause problems with performance intelligence quotient, academic achievement, memory, motor skills, and attention. Posterior fossa tumors are associated with memory and motor deficits (Ater et al. 1996). Tumors that involve the hypothalamic and parasellar region are related to growth hormone deficiency. Children treated with surgery alone for benign cerebellar lesions showed deficits in attention, memory, processing speed, and visual-constructive copying (Steinlin et al. 2003). Late effects associated with particular tumor locations are shown in Table 17.2 (Ellenberg et al. 1987; Lannering et al. 1990; Livesey et al. 1990; Mostow et al. 1991; Constine et al. 1993; Syndikus et al. 1994; Ilveskoski et al. 1997; Foreman et al. 1999).
Table 17.2
Central nervous system tumor locations and associated late effects
Region | Histology (percentage of primary CNS tumors) | Important associated late effects |
|---|---|---|
Supratentorial | Low-grade astrocytoma (15–20 %), high-grade astrocytoma (8–12 %), other glioma (5–10 %) | Poor cognitive function, poor manual dexterity, emotional difficulties, seizures, poorer overall quality of life |
Hypothalamic/parasellar | Craniopharyngioma (6–10 %), optic pathway glioma (4–8 %) | Growth hormone deficiency with hypothyroidism and hypogonadism |
Infratentorial | Primitive neural ectodermal tumor, cerebellar astrocytoma (12–15 %), high-grade pontine glioma (5–10 %), ependymoma (4–8 %) | Ataxia, primary thyroid dysfunction, ovarian dysfunction |
17.2.11.3 Genetics
Variation in patient response to treatment and neurocognitive outcomes may also depend on genetic polymorphisms. Currently, enzymes that effect chemotherapy metabolism and clearance are under investigation, including glutathione S-transferase and enzymes involved in folate metabolism. Signaling pathways known to be dysregulated in solid/brain tumors such as ErbB1-4, mTOR, IGF-IR, and PTCH1 are under investigation as future targets for therapy. In addition, identification of molecular markers predictive of outcome, survival, and treatment response may allow more patient-specific treatment plans in the future with the aim of cure and minimal late effects. In particular, radiogenomic studies have attempted to identify genetic variants underlying individual sensitivity to radiation in an effort to optimize radiotherapy for children genetically susceptible to neurotoxicity.
17.2.12 Treatment Factors
17.2.12.1 Radiation
The most common delayed toxicity of radiation therapy is cognitive impairment (Schiff and Wen 2006). The risk of neurocognitive late effects increases with the cumulative cranial radiation therapy dose given (Mulhern et al. 1998; Grill et al. 1999; Ris et al. 2001). Larger individual radiation fractions and larger fields of radiation also increase the risk of neurocognitive sequelae. A Pediatric Oncology Group study compared radiation doses and outcomes in medulloblastoma patients and found a decrease in neuropsychiatric toxicity in patients treated with 23.4 Gy instead of 36 Gy (Packer and Mehta 2002). Furthermore, a retrospective review of medulloblastoma patients indicated stable cognitive outcomes at 5-year follow-up in patients treated with lower dose craniospinal radiation and reduced volume tumor bed boost versus progressive decline in IQ in patients receiving standard craniospinal radiation or full volume posterior fossa boost (Moxon-Emre et al. 2014). An ongoing Children’s Oncology Group study sets out to prospectively measure cognitive changes in patients enrolled on therapeutic trials for medulloblastoma and CNS germinoma; both of these therapeutic trials are examining the feasibility of reduced doses of radiation in young, good risk medulloblastoma patients or chemotherapy sensitive germ cell tumors. The CNS tumor survivors who are treated with cranial radiation have greater deficits in neurocognitive functioning than those who do not receive cranial radiation. Neuropathologic changes following whole-brain radiation include leukoencephalopathy, mineralizing microangiopathy, subacute necrotizing leukomyelopathy, and intracerebral calcifications, commonly with subsequent cerebral atrophy and microcephaly.
17.2.12.2 Chemotherapy
Methotrexate, corticosteroids, carmustine, cisplatin, and cytarabine hydrochloride can be associated with long-term neurocognitive dysfunction. The most common neurocognitive effects of chemotherapy alone include deficits in visual processing, visual-motor functioning, and attention/executive functioning (Anderson and Kunin-Batson 2009). Methotrexate can cause a syndrome termed methotrexate leukoencephalopathy, most common in patients who have had treatment with both intravenous and intrathecal methotrexate therapy, in addition to whole-brain radiation therapy (Pizzo et al. 1979). Methotrexate has also has been shown to increase the effect of cranial radiation on the neurocognitive function (Waber et al. 1995; Moe and Holen 2000; Moleski 2000). Chemotherapy may have synergistic toxicity when given in combination with radiation therapy due to radiation-induced increase in blood-brain barrier permeability (Schiff and Wen 2006).
17.2.12.3 Surgery
A study of 28 children who were treated for medulloblastoma showed that neurologic deficits, meningitis, shunt infections, or the need for repeat surgery increased the risk of late neurocognitive deficits (Kao et al. 1994). In addition to neurocognitive affect, cerebellar dysfunction, spastic paresis, vision loss, epilepsy, and cranial nerve palsy may occur (Sønderkaer et al. 2003). Posterior fossa syndrome, characterized by loss of speech following surgery, is often present following midline posterior fossa tumor resection. Previously thought to be a transient phenomenon, posterior fossa syndrome has been associated with long-term neurologic and neurocognitive deficits (Turner et al. 2009).
17.2.13 Recommendations for Late Effects Care
In order to provide appropriate care for pediatric CNS tumor survivors, a plan for follow-up screening, surveillance, and prevention based on the individual’s cancer and treatment history as well as family history, lifestyle behaviors, and comorbid conditions should be established and communicated to the patient as well as their primary-care provider. Multiple studies have shown significant deficits in survivors’ knowledge regarding their diagnosis, completed treatment, and cancer-related health risks (Byrne et al. 1989; Hudson et al. 2002; Kadan-Lottick et al. 2002) as well as limited adherence with cancer screening guidelines (Nathan et al. 2010). It is essential that patients receive a summary of their treatment and appropriate recommendations for follow-up. Risk-stratified, lifelong medical monitoring can improve quality of life through early detection and intervention. Physicians also play a key role in encouraging health-promoting behaviors and prevention strategies. In order to properly address the neuropsychological deficits in CNS tumor survivors, a comprehensive neuropsychological assessment upon entry to a late-effects or follow-up clinic is necessary, regardless of patient or family report of deficits. For providers of late-effects or follow-up care for CNS tumor survivors, it is important to consider that many insurance companies may not provide coverage for neuropsychological testing (Oeffinger et al. 2008).
There is controversy regarding the optimal setting for pediatric cancer survivor follow-up. Some pediatric cancer survivors may have minimal risk for late effects and could receive follow-up by a local primary-care physician with guidance and support from a pediatric oncology treatment group. Pediatric brain tumor survivors, however, are at high risk for a large variety of significant late effects and would be best served by continued follow-up through adulthood at a multidisciplinary late-effects clinic at a center that provides pediatric oncology care. The Children’s Oncology Group is a group of over 240 institutions that has developed “Long-Term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young-Adult Cancer,” available at www.survivorshipguidelines.org.
17.3 Palliative Care
Cancer is the leading disease-related cause of death in children aged 5–14 years (Murphy et al. 2013). The CNS malignancies are the second most common type of cancer diagnosed in children (Howlader et al. 2014). Surveillance, Epidemiology, and End Results (SEER) registry data show 5-year relative survival rate of brain and other nervous system malignancies in children aged 0–19 from 2004 to 2010 to be 74 % (Howlader et al. 2014). These mortality data define the important role of pediatric palliative care in the management of pediatric CNS malignancies.
The World Health Organization defines palliative care for children as an active and total approach to care, embracing physical, emotional, social, and spiritual elements (Waldman and Wolfe 2013). This approach focuses on enhancing patient’s quality of life and support for family by managing distressing symptoms and providing respite and care through treatment, death, and bereavement. Care can be provided in multiple locations including the hospital, a hospice facility, or within the child’s home. The family-centered approach requires a multidisciplinary team. Optimally, palliative care is not separate from curative care and should be integrated into the overall care plan from the point of initial diagnosis for patients with a life-threatening illness, but instead is increasingly incorporated into treatment planning as prognosis for cure becomes less likely (Fig. 17.3). Ideally, the need for palliative care should be based on the prognostic uncertainty inherent to a cancer diagnosis rather than likelihood of survival.
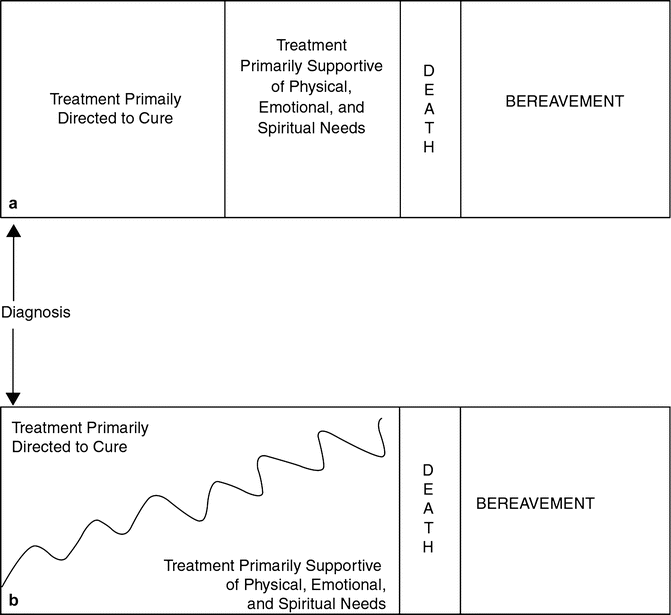

Fig. 17.3
Models for incorporating palliative care. (a) Palliative care is introduced only after treatment directed to cure is completed. (b) Palliative care is introduced early on during treatment directed to cure and begins to comprise a larger component of care over time (Sahler et al. 2000) (Reproduced with permission from Pediatrics, ©2000 by the AAP)
Concerns regarding growth and development uniquely separate pediatric palliative care from adult palliative care. Developmental differences among infants, children, and adolescents must be considered when designing and implementing a pediatric palliative-care program. In addition, pediatric palliative care encounters different obstacles than adult palliative care (Korones 2007). Overall, only 25 % of childhood deaths are from a complex chronic medical condition and therefore suitable for involvement of a pediatric palliative-care team. When combined with the overall lower mortality rate in children, this creates a relatively small population of pediatric patients compared to the adult population utilizing palliative-care services. On average, a general pediatrician in North America cares for less than three children who die per year. Limited experience contributes to physician discomfort when providing pediatric palliative care (Kolarik et al. 2006).
The Medicare hospice benefit is primarily targeted for adults and requires patients to forgo curative or life-prolonging therapy. Pediatric palliative care strives to integrate curative and palliative care in combination, and thus, this reimbursement approach often directly conflicts with care plans. Typically, pediatric care does not require a do-not-resuscitate order or prognosis for short-term survival. Under the US Affordable Care Act, children under the age of 21 eligible for Medicaid or the Children’s Health Insurance Program and diagnosed with a life-limiting illness may receive all services related to the treatment of that illness; this includes palliative-care and hospice-care services provided concurrently with other disease-related treatments (Waldman and Wolfe 2013). Pediatric providers and families often choose to continue supportive measures such as blood transfusions and supplemental feeding with the goal of contributing to the overall well-being of the child (Sirkia et al. 1997).
Inadequate training is another barrier to providing good palliative care. In a survey of medical staff, 49–54 % of attending physicians and residents responded that they felt inexperienced in providing pain management for dying patients (Contro et al. 2004). Multiple studies have documented a lack of pediatric palliative-care education during medical school and residency (Flint and Weidner 2012). Pediatric patients have unique medical and psychosocial needs that adult-trained palliative-care providers may feel unequipped to address. Limited clinical and community resources for hospice, homecare, and pediatric end-of-life services further complicate efforts to maximize quality of care outside of the hospital.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree