Figure 12.1 Keratitis isolates (1993–2006).
Clinical features
The presenting symptoms, clinical history, and exam findings may suggest an infectious keratitis but are seldom pathognomonic for a particular organism. The presenting signs of bacterial keratitis vary depending on the virulence of the organism, duration of infection, structural status of the cornea, and host inflammatory response.
Common presenting symptoms include pain, decreased vision, tearing, and photophobia. Eyelid edema, conjunctival hyperemia with a papillary reaction, and chemosis are typical findings. A corneal epithelial defect with adherent mucopurulent exudate and underlying stromal infiltrate is a hallmark sign for infectious keratitis (Figure 12.2). Multiple focal infiltrates can be seen with contact lens use or with polymicrobial infections. Migration of inflammatory cells causes a diffuse cellular infiltration adjacent to and within the ulcerated stroma. An anterior chamber reaction can range from mild cells and flare to a marked hypopyon (Figure 12.3). A cornea damaged from prior disease can present with less distinct signs and symptoms. Pre-existing corneal scars, epitheliopathy, or inflammation confuse the picture as does prior use of antibiotics and corticosteroids. On examination, all ocular abnormalities should be documented in detail to help track the clinical course on subsequent visits. Repeat measurements of the size of the epithelial defect, the depth of the stromal infiltrate, and the severity of inflammation can be used to assess the effectiveness of treatment.
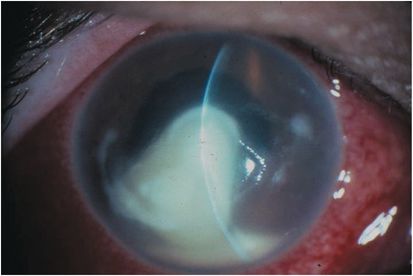
Figure 12.2 Infectious keratitis.
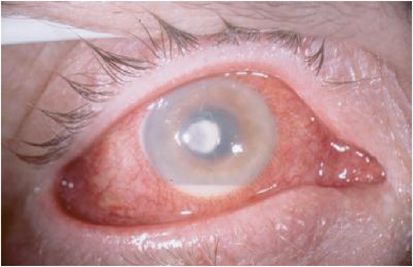
Figure 12.3 Hypopyon.
Nontuberculous mycobacterial keratitis has been reported with increasing frequency after LASIK, including several clusters of cases. In two recent reviews of post-LASIK corneal infections, Mycobacterium represented the most common etiologic organism. The isolated subtypes include the fast-growing Mycobacterium chelonae, Mycobacterium abscessus, Mycobacterium fortuitum, and Mycobacterium mucogenicum, as well as the slow-growing Mycobacterium szulgai. Nontuberculous keratitis after LASIK is characterized by a delayed onset with an indolent course. Time of onset from fast-growing organisms averaged 3.4 weeks after the procedure, whereas the slow-growing M. szulgai can present 6 to 24 weeks after surgery. Symptoms can range from a mild foreign-body sensation to pain, redness, photophobia, and decreased vision. The infiltrate, which can be multiple, begins in the interface and spreads to adjacent stroma of the flap and stromal bed. Anterior perforation through the flap can occur with progression of infection. The location can be central, paracentral, or peripheral. In addition to a focal infiltrate, a cracked windshield appearance of infectious crystalline keratopathy has been reported (Figures 12.4A and B).
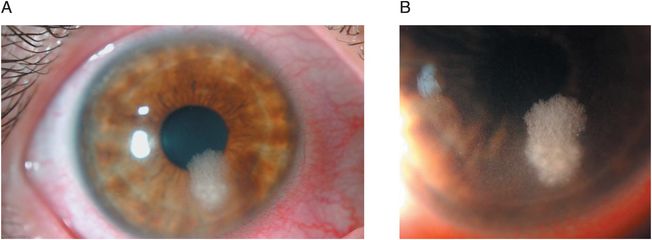
Figures 12.4 (A and B) Infectious crystalline keratopathy.
Diagnostic techniques
Currently, routine culture of corneal infections is not the usual practice in the community. A small peripheral ulcer may be treated empirically, but a large, purulent, central ulcer that extends to the middle to deep stroma should be cultured. In addition, ulcers that are atypical or clinically suspicious for fungal, mycobacterial, or amebic infections or are unresponsive to initial broad-spectrum antibiotics warrant cultures. Topical anesthesia with proparacaine hydrochloride is preferred because it has fewer antibacterial properties than other topical anesthetics that might interfere with culture yields. A sterile platinum spatula is used to scrape the leading edge as well as the base of the ulcer while carefully avoiding contamination from the lids and lashes. Organisms such as Streptococcus pneumoniae are more readily recovered from the ulcer edge, whereas other organisms such as Moraxella are characteristically recovered from the base. The scrapings are inoculated onto solid media (blood, chocolate, and Sabouraud’s agar) by streaking a row of Cs. New material is recovered for each row. Scrapings are also placed on microscope slides and stained with Gram and Giemsa stains. Special stains include Ziehl–Neelsen acid-fast stain for Mycobacterium, Actinomyces, and Nocardia. Acridine orange is a fluorescent dye that may be helpful in identifying bacteria when yields are low, but this stain does not yield classification information that Gram stain provides.
In vivo confocal microscopy may be helpful in atypical, nonbacterial corneal infections where the organisms are larger and not as amenable to culture or identification. In cases of deep stromal suppuration that is not readily accessible or a progressive microbial keratitis unresponsive to therapy, a corneal biopsy may be warranted. A round 2-mm to 3-mm sterile disposable skin trephine is used to incise the anterior corneal stroma, and lamellar dissection is performed with a surgical blade. The specimen is then ground in a mortar with trypticase soy broth and plated on media.
Treatment
Routes of administration
The topical application of drugs with eyedrops is the preferred method of treatment of bacterial keratitis. Increased drug penetration can be achieved by higher concentrations, more frequent applications, and by the typical presence of an epithelial defect. Fortified antibiotics are made by mixing the powdered drug or diluting the parenteral form with artificial tears or balanced salt solution. These freshly prepared solutions remain stable for up to a week without significant loss of activity. Although ointments prolong corneal contact time and lubricate the ocular surface, peak corneal concentrations may be limited when compared with solutions. They also retard the absorption of antibiotics delivered in eyedrop form. Ointments can be used as adjunctive therapy at bedtime in less severe cases.
The need for other routes of antibiotic administration in bacterial keratitis is rare. Subconjunctival injections may not have a therapeutic advantage over topical solutions. However, they may be indicated in certain clinical situations such as imminent perforation or spread of infection to adjacent sclera, especially when patient compliance is an issue. Soft contact lenses and collagen shields can act as drug depot/delivery devices and aid in sustaining high corneal drug levels. These “bandage” contact lenses may also provide protection to promote reepithelialization. Systemic therapy is indicated for gonococcal infections as well as for young children with severe H. influenzae or P. aeruginosa keratitis. Systemic antibiotics are also indicated for perforations and scleral involvement.
Empiric therapy
Because bacterial keratitis can rapidly progress and threaten vision, treatment should begin when an infectious process is suspected to limit further damage in an effort to preserve vision. Topical broad-spectrum antibiotics are initially used and later modified according to culture results, antibiotic susceptibilities, and clinical response. For severe cases, combination therapy with fortified β-lactam (cefazolin 50 mg/mL) and aminoglycoside (tobramycin or gentamicin 14 mg/mL) (Figure 12.5) provides adequate coverage of both gram-positive and -negative organisms that cause bacterial keratitis. Vancomycin (50 mg/mL) can be substituted for cefazolin in cases of penicillin allergy or resistance in Staphylococcus species. Because of a high prevalence of methicillin resistance in some centers, vancomycin instead of cefazolin is utilized as a first-line agent. A loading dose is achieved with a drop every 5 minutes for five applications. Antibiotic is then continued every 30 minutes to 1 hour around the clock for several days and then rapidly tapered once control of the infection has been achieved.
Figure 12.5 Topical aminoglycosides are combined with β-lactam therapy.
Single-agent therapy with fluoroquinolones has previously been shown to be as effective as combination therapy in treating bacterial keratitis. The widespread systemic use of the second (ciprofloxacin and ofloxacin) and third (levofloxacin) generation fluoroquinolones has, however, led to the emergence of resistance in several bacterial species, including Pseudomonas aeruginosa. The fourth-generation fluoroquinolones, gatifloxacin and moxifloxacin, have been developed to improve coverage of gram-positive and atypical mycobacterial pathogens. They require two mutations to establish resistance and, therefore, are more effective against gram-positive organisms that already have a single mutation and are resistant to older generation fluoroquinolones. Unfortunately, large surveys have noted that the vast majority of methicillin-resistant Staphylococcus aureus is also resistant to all currently available topical ophthalmic fluoroquinolones.
Regardless of the susceptibility profile, a favorable response to empiric therapy merits continuing the treatment. Positive signs of clinical improvement include decreased pain and discharge, consolidation of the stromal infiltrate, decreased anterior chamber reaction, and corneal reepithelialization. Culture and antibiotic susceptibility results can be used to focus therapy against the offending organism or to discontinue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree