Fig. 5.1
Matched endoluminal CTC (left panel), white-light endoscopic (middle panel) and narrow-band imaging endoscopic (right panel) images of a tumour in the ascending colon, just above the ileocecal valve. This was macroscopically a laterally-spreading tumour, granular type (LST-G) and was removed by endoscopic mucosal resection (EMR). Histologically, the lesion was a tubulovillous adenoma with high-grade dysplasia and a small focus of invasive adenocarcinoma, which had been completely excised endoscopically
Colorectal neoplasia occurs on a continuum, from benign, low-risk tubular adenomas that are destined to cause no harm (and indeed may even regress spontaneously [20]), via intermediate- and high-risk adenomas, through to frankly malignant invasive polyps and masses [21]. This, of course, is the rationale for CRC screening—removal of benign but potentially pre-malignant precursor lesions ultimately reduces subsequent CRC incidence [22–25] (and early detection and treatment of established cancer reduces disease mortality). However, polyps do not cause symptoms unless very large. Detection of small (6–9 mm) and diminutive (0–5 mm) polyps is therefore of considerably less importance for symptomatic patients than for screening populations, as these will undoubtedly be incidental to the index clinical presentation. It could be argued that each colonic examination has the potential to opportunistically detect and remove precursor lesions, and so their detection is an important facet of thorough testing. It must be remembered, however, that the large majority of polyps will never develop into CRC—over 40% of adults aged over 50 years have adenomas but only 5% will ever develop CRC [26]. Clearly, polyps with the highest risk of progressing to CRC are the most important to detect, and it has long been known that risk goes hand-in-hand with polyp size [21]. Since both meta-analysis [17] and more recent multicenter prospective cohort studies [27] show that CTC has sensitivity of approximately 90% for colorectal neoplasia of 10 mm or more, the technique seems well-suited to this role.
Our knowledge regarding the use of CTC for symptomatic patients, and in particular its relationship with colonoscopy, has been clarified significantly by the UK SIGGAR (Special Interest Group in Gastrointestinal and Abdominal Radiology) studies [28]. These parallel, multicenter, pragmatic randomized controlled trials were designed to compare CTC with the radiological alternative, barium enema [29]; and to compare CTC with colonoscopy [30]. It is important to note that the goals and primary outcomes of the two trials were different. The CTC vs. barium enema trial was designed to achieve 90% power to detect a difference in detection rates between the two tests for CRC and large (≥1 cm) polyps, assuming that CTC detected roughly 30% more CRC and large polyps than barium enema. 3838 patients were randomized 2:1 in favor of barium enema [31]. A significant difference was indeed demonstrated—CTC had a significantly higher detection rate than barium enema (relative risk 1.31, 95%CI 1.01–1.68), and missed fewer cancers at 3 year follow-up (3 missed of 45 detected vs. 12 missed of 85 detected) [29]. The authors concluded that barium enema should be abandoned as a test for colorectal neoplasia in favor of CTC.
Conversely, the CTC vs. colonoscopy trial was not designed or powered to compare the detection rates of the two tests. As mentioned above, meta-analysis suggests that the two have near-equivalent sensitivity for established CRC [17, 18], and that CTC is 90% sensitive for large polyps when compared to colonoscopy or colonoscopy with segmental unblinding [17]. Accordingly, a trial powered to detect a difference in detection rates between colonoscopy and CTC would have required tens of thousands of patients.
Instead, the trial was designed to assess a different issue relevant to the clinical implementation of both CTC and colonoscopy—the need for further testing after the index procedure. Both CTC and colonoscopy can generate further tests; CTC cannot remove polyps or biopsy cancers, and colonoscopy may be incomplete (and detected cancers also require CT staging). If a significant proportion of patients having CTC to investigate colorectal symptoms require subsequent colonoscopy in any case, there is little point in them being subjected to CTC up-front, which would add inconvenience, risk and cost for no benefit. Therefore, the SIGGAR CTC vs. colonoscopy trial was designed to compare the referral rate for additional colonic investigation; the detection rate of CRC and large polyps was a key secondary outcome, as was the rate of subsequent (missed) colorectal cancer after 3 year follow-up. 1610 patients were randomized (1072 to colonoscopy and 538 to CTC), of whom 30 withdrew consent, leaving 1580 for analysis (colonoscopy: 1047; CTC: 533).
Overall, 160 (30.0%) of patients in the CTC arm had an additional colonic investigation compared with 86 (8.2%, p < 0.0001) in the colonoscopy arm. The majority of the referrals for an additional colonic test after CTC were to investigate a suspected cancer or large polyp (83 patients, 15.6%). Of these, 51 (61%) had CRC or a large polyp confirmed. A further 49 patients (9.2%) randomized to CTC were referred for an additional test to investigate smaller polyps (i.e. 9 mm or less)—only 3 (6.1% of the 49) of these ultimately had a large polyp, and none had cancer. Finally, 28 participants (5.3%) randomized to CTC were referred on as a result of diagnostic uncertainty; a single patient in this category had a ≥1 cm polyp, and, again, none had CRC. Therefore, of the 77 patients referred for a further test where CTC was either uncertain or showed small polyps only, none had cancer and only 4 (5.2%) had a polyp measuring ≥1 cm. The situation after colonoscopy was somewhat different; of the 86 patients requiring further testing, the majority (73; 7.0%) were referred because of clinical uncertainty after the (attempted) colonoscopy, primarily because of failure to intubate the caecum. Of these, three had CRC proven subsequently (4.1% CRC rate).
As noted above, a key secondary outcome of the study was the detection rate of CRC and large polyps (pooled). Here, no significant difference was detected between the two tests; 119 (11.4%) for the 1047 patients randomized to colonoscopy versus 57 (10.7%) for the 533 randomised to CTC (p = 0.69). Detection rates of CRC were near-identical between the two tests; 58 (5.5%) in the colonoscopy arm and 30 (5.6%) in the CTC arm. After a minimum of 3 years follow-up via cancer registries, no additional CRC were reported in the colonoscopy group, and just 1 CRC was identified in the CTC arm (miss rate of 3.4%).
Taken together, the results of the SIGGAR CTC vs. colonoscopy trial showed that CTC had a higher referral rate for onward testing than colonoscopy, but that most of these referrals were to confirm the presence of a suspected polyp. Subsequent diagnosis of clinically-significant lesions (CRC or ≥1 cm polyps) was rare if index CTC found only small polyps or where the radiologist was uncertain. Missed cancer presenting within 3 years was rare for CTC (a single case in the CTC vs. colonoscopy trial and three cases in the CTC vs. barium enema trial, for an overall rate of 5.4% across both trials), and there was no significant difference in detection rates between CTC and colonoscopy. Although the study was not powered to detect a significant difference, this finding (and the point estimates of CRC and large polyp detection rates) does suggest that if there is a difference, it is likely to be small.
It may be somewhat surprising that only 51 of the 83 patients (61%) referred for onward colonic testing to investigate CRC or large polyp suspected at CTC were ultimately diagnosed with such a lesion; i.e. the positive predictive value (PPV) was moderate. This was primarily due to CTC false-positives, as size mismatching was rare (i.e. a polyp measured as over ≥1 cm at CTC, but found to be smaller at colonoscopy). The explanation for false-positives is likely twofold. Firstly, the study was conducted in an era when oral contrast faecal tagging was not yet mandatory. Oral contrast is used to “label” stool and liquid residue with dense iodine or barium-based compounds, which can then be distinguished from polyps by virtue of increased radiodensity at CTC. This prevents a common cause of false-positive diagnoses at CTC, namely residual stool, while simultaneously helping detect polyps that would otherwise be submerged and obscured (Fig. 5.2). Secondly, radiologists knew they were being studied, and that CTC was being evaluated. This may have prompted them to “err on the side of caution” and to flag even equivocal findings as potentially positive, thereby reducing the chance of missed lesions (but potentially increasing false-positives). Irrespective of the reason, because the prevalence of CRC and large polyps was relatively low (11%), even high test sensitivity and specificity can result in moderate-to-low PPV, as was observed. For example, at a prevalence of 11%, and postulating a sensitivity and specificity both of 93%, PPV is only 62%, similar to that observed in the SIGGAR CTC vs. colonoscopy trial. Therefore, the findings were entirely consistent with existing literature and meta-analysis.
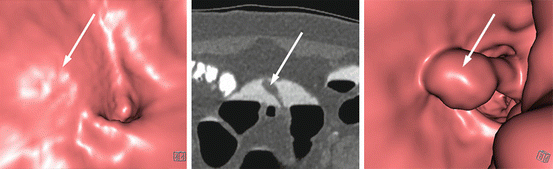

Fig. 5.2
Endoluminal image shows only a fluid level (arrow in left panel). The two dimensional image shows there is a pedunculated polyp (arrow in middle panel) which is submerged in oral contrast-tagged fluid. Tagged datasets can be interrogated endoluminally by using electronic stool subtraction (“electronic cleansing”), as in the right panel (arrow shows the head of the polyp), although these often cause digital artifacts that can impeded interpretation
To summarize, the diagnostic accuracy of CTC for symptomatic patients is sufficiently high to advocate its use for the clinically-relevant target of CRC and ≥1 cm polyps. Meta-analysis of cohort studies, and now level 1 randomized trial data, confirm excellent sensitivity can be generalized across a wide range of sites. CTC detects important colorectal neoplasia at a similar rate to colonoscopy, although it is important to establish clear referral guidelines to avoid unnecessarily high rates of subsequent colonoscopy.
5.3.2 Extracolonic Detection
Abdominal symptoms are often vague and the organ of origin may be obscure, often beyond the colorectum. Therefore, the ability to interrogate structures outside the gastrointestinal tract at the same time as a high-quality examination of the colorectum may be advantageous for symptomatic patients. Furthermore, it may permit serendipitous discovery of unrelated (but clinically-important) pathology. Conversely, extracolonic detection may be a disadvantage if it precipitates further tests (with the associated costs, inconvenience and risks that these entail) for incidental findings that transpire to be of no clinical importance ultimately. CTC is readily able to depict extracolonic pathology in the torso from the lower chest to the bottom of the pelvis, since CT scanning of this region is a fundamental requirement of the technique (Fig. 5.3). Conversely, colonoscopy rarely detects extracolonic disease unless it spreads directly into the colon (for example, extrinsic involvement by serosal tumour, typically arising from the gynaecological tract; or by endometriosis) or involves it as part of a multiorgan process (e.g. vasculitis or amyloidosis), and rarely even then. In a symptomatic setting, where the colon is normal, the patient still has a problem that caused them to seek medical attention, and, at least in some cases, the next step would be to investigate the other abdominopelvic viscera, and CTC neatly combines these aspects in a single examination.
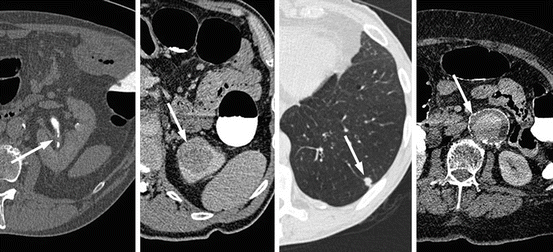

Fig. 5.3
Selected axial images from CTC examinations depicting incidental important extracolonic pathology; from right to left, left urothelial thickening (transitional cell carcinoma), a 3.5 cm left upper pole renal mass (renal cell carcinoma), a 1 cm left basal pulmonary nodule (non-small cell lung carcinoma) and a 4.8 cm infra-renal abdominal aortic aneurysm
Although seemingly intuitive, this approach is not necessarily of clinical benefit. Not all symptoms or signs suggestive of CRC require extracolonic evaluation if colorectal investigation has been negative. Genuine bright red rectal bleeding is very rarely due to extracolonic pathology; iron-deficiency anaemia should initially provoke assessment of the upper gastrointestinal tract rather than the other abdominopelvic viscera; and so on. Although observational data provide some useful information confirming that CTC can and indeed does depict extracolonic pathology, including neoplasia [32], the clinical trajectory of such diagnoses in comparison to the default (colonoscopy) is largely unknown with such study designs, particularly for symptomatic patients. Randomized data from the paired SIGGAR trials [33] help address this issue, since this avoids the biases inherent to other designs.
Taken together, at least one previously unknown extracolonic finding was diagnosed by CTC in 959 (58.7%) of 1634 patients (excluding those patients with CRC). For the most part, extracolonic findings were unimportant, and did not merit further diagnostic investigation. However, 136 (8.3%) patients having CTC ultimately did have an extracolonic finding investigated (or treated); approximately half of these investigations were non-invasive imaging only, with the remaining half being an invasive procedure or surgery (with a roughly equal split between the two). Surgery was sometimes to combine treatment with diagnosis (for example, excision biopsy); the commonest surgical procedures were nephrectomy, oophorectomy (with or without hysterectomy) and aneurysm repair. Extracolonic diagnosis were judged to explain the patient’s presenting symptoms in only 3–4% of patients overall. Ultimately, 25 patients (1.5%) having CTC were diagnosed with extracolonic malignancy. Conversely, no patients undergoing colonoscopy required evaluation for extracolonic pathology, and only 42 of 2223 patients (1.9%) having barium enema had one or more extracolonic finding reported. Five patients (0.2% of the total) ultimately received an extracolonic diagnosis, of which three were malignant. A further 14 patients having CTC had aortic aneurysms diagnosed, compared to none for barium enema or colonoscopy.
At first sight, therefore, this appears to be a clear benefit for CTC—1.5% of patients having CTC received a diagnosis of extracolonic malignancy, compared with 0.13% for barium enema and none for colonoscopy. However, this difference was short-lived; by 1 year, using cancer registry data, there was no difference in the diagnosis rates of extracolonic malignancy irrespective of the initial randomized procedure. This is surprising—how are patients having barium enema or colonoscopy receiving a diagnosis of extracolonic malignancy? There are several possible explanations; firstly, not all malignancies can be diagnosed by CTC—early cross-sectional abdominopelvic imaging can only ever affect diagnosis rates for certain primary tumours (particularly renal, ovarian and pancreatic). Since the commonest malignancies are those of the lung, breast and prostate, none of which are readily diagnosed by CTC, it is implausible that CTC accelerates their diagnosis, diluting the effect of early CT. Indeed, in the SIGGAR trials, 58% of cancers diagnosed within 1 year of randomization to CTC had not been diagnosed by CTC itself; either because they were outside of the field of scanning or were occult (or overlooked) at that time. Secondly, and perhaps most pertinent, it is highly probable that patients randomized to barium enema or colonoscopy underwent cross-sectional imaging subsequently as the search for the cause of their symptoms continued. As noted above, even after normal colonic investigation, patients may still have symptoms, provoking their physician to investigate further. This is with good reason—incidence rates of extracolonic malignancy in the SIGGAR trials were double that of the general population (matched for age and sex)—abdominal symptoms are clearly an important flag and further investigation is warranted.
Therefore, although CTC permits rapid diagnosis of some extracolonic malignancies, there is no difference in the rate of such diagnoses by 1 year, when compared with colonoscopy. It is likely that this is partly because some cancers are not diagnosable by CTC, and partly because colonoscopy is often followed up by additional abdominopelvic cross-sectional imaging (namely CT). This has considerable implications for the cost-effectiveness of the two tests, and it is to this aspect that we turn next.
5.3.3 Cost Effectiveness
Most studies of the cost-effectiveness of CTC have described models rather than actual data observed in a trial. Furthermore, most models have compared the cost effectiveness of CTC versus alternatives in a screening context. The situation for symptomatic patients is different—prevalence of both colonic and extracolonic abnormality is much higher, implying that CTC is far more likely to be followed by confirmatory or therapeutic colonoscopy in a symptomatic setting versus screening. The goals of investigation are also rather different. Screening aims to (a) reduce CRC incidence (by prophylactic polypectomy, thereby interrupting the adenoma-carcinoma or serrated pathways to carcinogenesis) and (b) decrease CRC mortality by early detection, thereby increasing the likelihood of curative treatment. The cost-effectiveness of screening is largely contingent on improving these outcomes and may even be cost-saving because disease becomes less common, and is easier and cheaper to treat if it does occur. Conversely, investigation of symptomatic patients aims to explain symptoms and exclude serious causes. By the time CRC is symptomatic, it is on average more advanced than CRC detected by screening; and disease prevention is less effective because patients are typically older and have more co-morbidity, meaning they may not have sufficient life expectancy to benefit from prophylactic polypectomy. Accordingly, symptomatic and screening scenarios require separate cost-effectiveness models.
Clearly, such models are highly dependent on the inputs, which include estimates for the frequency of tests (which depend on study setting and healthcare systems from which they are derived) and their unit costs. Unit costs of both CTC and colonoscopy vary widely internationally, as do the downstream trajectory and costs of future clinical activity (e.g. further tests, outpatient attendances, surgical procedures, hospitalization etc.). Most models have utilized estimates drawn from a wide range of primary research and so these estimates differ widely. However, it is well-recognised that the most accurate models use estimates drawn directly from observations obtained directly from a pragmatic clinical trial, since these best reflect what happens in “real life”. Again, the most robust data to date are from the SIGGAR trials, which were pragmatic (i.e. designed to be representative of routine clinical practice), included a range of differing hospitals (21 in total), and health economic data were collected prospectively with the intention of a cost-effectiveness analysis. The results were surprising: Although the unit cost of CTC is considerably lower than that for colonoscopy (and subsequent testing was far higher for patients randomized to CTC), once downstream costs were considered, the difference between the two tests virtually disappears; total costs were £651 per patient for colonoscopy and £627 for CTC, a difference that was not statistically significant. Since referral rates for colonoscopy after CTC was a surprisingly high 30%, costs would move further in favor of CTC if referrals could be decreased. Additionally, for CTC specifically, roughly half of the overall per-patient costs were due to downstream costs (primarily, confirmatory colonoscopy or investigation of extracolonic findings). Since colonoscopy did not precipitate any immediate extracolonic investigation, downstream costs were much lower. However, the preceding section makes it clear that patients having colonoscopy are very likely having CT scanning or similar within 1 year of initial referral, representing costs that were not captured in the original trial data: costs beyond the immediate diagnostic episode were not captured. If true, this will increase the downstream costs of colonoscopy very considerably, and so reduce its cost-effectiveness relative to CTC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree