20 Intratumoral Chemotherapy
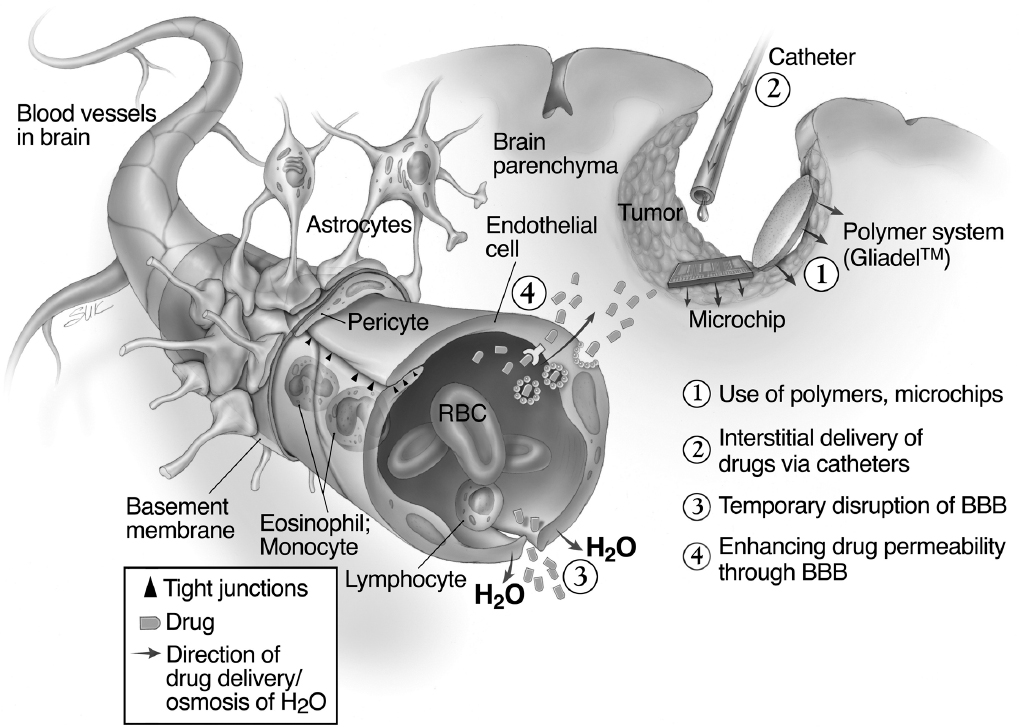
The infiltrative nature of malignant glioma is one of the greatest hurdles to effective therapy. These tumors are primarily a regional disease, and 80% of recurrences are within 2 cm of the initial resection field.1 Thus, improving local disease control may significantly affect overall survival. Surgery is limited by the increased risk of removing tumor-infiltrated functional brain tissue, resulting in significant neurologic sequelae. Similarly, doses of radiation that would be optimal for tumor eradication may cause damage to the surrounding brain. Thus improving chemotherapeutic control of these tumors is necessary to improve overall survival. Physiological and pathological barriers within the central nervous system (CNS) limit the efficacy of systemic chemotherapy for brain tumors. As a result, systemic administration of antineoplastic agents by oral or intravenous routes has failed to achieve effective drug concentrations into the target site even at systemically toxic doses. A major limiting factor for adjuvant systemic therapies is the blood–brain barrier (BBB), which restricts entry of drugs into the brain.
Several approaches to improving drug delivery have been investigated (Fig. 20.1). This chapter focuses on the options to overcome the limitations of systemic therapy by using local delivery. The goal of these therapies is to provide sustained tumoricidal doses of drug intratumorally or peritumorally while avoiding systemic toxicity. Three systems have been utilized clinically: direct injection, convection-enhanced delivery (CED), and implantation of a drug-loaded polymeric matrix within the tumor.
 Direct Injection
Direct Injection
Direct injection of drug, into either the tissue or the ventricle, was one of the earliest local delivery systems. Catheter implants into the tumor cavity or ventricle, connected to an Ommaya reservoir, have been used to deliver intermittent bolus injections of both chemotherapeutic2–4 and biological agents.5,6 There are anecdotal case reports indicating successful outcomes using the Ommaya system or direct injection of various agents, but there have been no successful large-scale clinical trials proving their efficacy. One main limitation to these systems is poor drug distribution into solid brain or tumor tissue. With small molecules, depth of distribution with a concentration-based gradient is often limited to several millimeters, with an exponential decay in concentration from the point source. Thus, distribution of therapeutic concentrations of drug is limited to a small volume of tissue around the injection site, often with high and sometimes toxic concentrations of drug at the point source.
Special Consideration
• Direct infusion limits the distribution of drug to within a few millimeters of the injection site.
Direct injection is being clinically used for the distribution of carmustine (bischloroethylnitrosourea [BCNU]) dissolved in absolute ethanol (DTI-015).5 Preliminary studies using direct injection of viral agents have been performed with adenoassociated virus containing p53 transcript or the herpes simplex virus thymidine kinase gene. Unfortunately, studies have shown good local distribution and gene expression in the injected tissue at distances of only a few millimeters.7,8
 Convection-Enhanced Delivery
Convection-Enhanced Delivery
Convection-enhanced delivery is a drug delivery method in which macromolecules are distributed into brain parenchyma using a positive pressure gradient. Whereas diffusion uses a concentration gradient to distribute molecules, the use of a pressure gradient facilitates the distribution of a homogeneous concentration of small and large molecules over large distances by bulk displacement of the extracellular fluid with the infusate. The therapeutic agent is delivered into the parenchyma via a microcatheter inserted into the tissue with infusion rates of 0.5 to 10 µL/min. The distribution from a single point source creates an elliptical to spherical distribution with 2 to 3 cm diameter, resulting in a linear relationship between infusion volume and distribution volume. Distribution of macromolecules by CED results in a large improvement over conventional forms of drug delivery. For example, 200 µL of radiolabeled albumin injected into the brain distributes to less than a 2-mm radius into the adjacent brain tissue after 4 hours (diffusion), whereas microinfusion of the same volume of albumin results in distribution of 1.5 cm radius.9,10
The efficacy of CED is based on the half-life, surface characteristics, and size of the macromolecules to be delivered, as well as the delivery catheter and infusion characteristics. Convection-enhanced delivery into gray and white matter has shown reproducible distributions to a large volume of tissue with homogeneous drug concentration of macromolecules, including immunoglobulin G (IgG) (180 kd).9,10 A key element for effective large-scale distribution of a molecule or nanoparticle is a stable half-life of the agent in the extracellular space. The distribution of lipophilic molecules such as BCNU is severely limited by transvascular export through blood vessels leading to a high efflux of drug. Other molecules may be prone to degradation from peptidases located in the extracellular space of the brain parenchyma.
Another critical determinant for distribution is the surface characteristic of the macromolecule. Binding of the agent to extracellular matrix or surface receptors may limit distribution of drug. Although binding to cell surface receptors may be overcome by saturating receptor binding, adherence to heparin sulfate proteoglycan in the extracellular matrix has limited distribution of growth factors.11 Co-infusion of heparin or fibroblast growth factor has overcome this limitation and enables reproducible large volumes of distribution.12
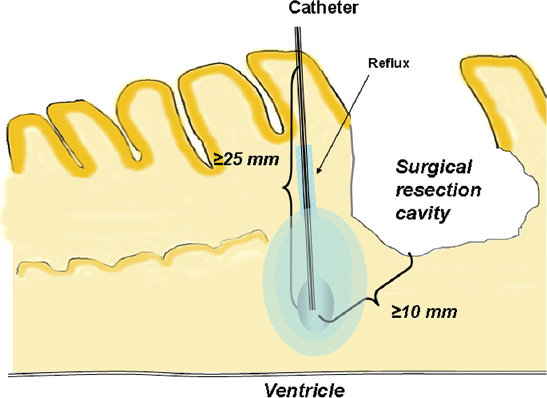
The size of the molecule also affects volume of distribution. Initially, 180 kd was felt to be the upper limit to pass through the extracellular space. Recently, adeno-associated virus (40 nm) and liposomes (50 to 200 nm) have been distributed to large volumes of brain tissue.13,14 Both agents, however, require modification of the surface (pegylation with liposomes and heparin co-infusion to saturate heparin sulfate proteoglycan binding with adeno-associated virus). In addition, the volume of distribution is also affected by retrograde movement of fluid along the outside of the catheter (back-flow) (Fig. 20.2). The distance of retrograde flow along the catheter is determined by catheter diameter, infusion rate, and tissue density. The larger the diameter of the catheter, the greater the backflow along the catheter. If the retrograde flow of fluid around the catheter reaches low pressure zones (necrosis, cerebrospinal fluid [CSF] space), the fluid will inadvertently be lost into the CSF space. This leads to the accumulation of drug in the region of necrosis, or loss of drug into the intrathecal space. Finally, increasing the infusion rate can increase the overall volume of distribution; however, this will also increase the backflow distance, potentially shunting fluid away from the target volume. Parameters affecting the distribution of infusate are described in the text box below.
Although the physical parameters influencing optimal drug delivery by CED have not been finalized, the potential of obtaining significantly high concentrations of a therapeutic agent over large volumes of brain tissues has led to several clinical trials in patients with neurodegenerative disorders and malignant gliomas. Therapeutic studies for malignant gliomas have focused on distributing targeted macromolecules (chimeric toxins) or currently available small-molecule drugs. A phase 1 study of CED of topotecan consisting of 16 patients established a maximum tolerable dose of 0.1 mg/mL.15 A phase 2 study of intratumoral CED of paclitaxel showed some preliminary positive results but was associated with some nonspecific toxicity including meningitis, infection, and transient neurologic deterioration.16 More recently, CED of topotecan was used in two pediatric patients with diffuse intrinsic pontine gliomas, and this study demonstrated that CED was possible for brainstem tumors but that only lower infusion rates were tolerated (< 0.4 mL/h).17 Some ongoing studies are designed to de-escalate the drug concentration.
Key Convection-Enhanced Delivery Parameters Affecting Infusate Distribution
• Catheter
 Configuration
Configuration
 Location of port
Location of port
 Size
Size
 Trajectory
Trajectory
 Depth of positioning
Depth of positioning
• Drug
 Surface characteristics
Surface characteristics
 Tissue half-life
Tissue half-life
 Size
Size
• Target tissue
 Microanatomy
Microanatomy
 Tissue density
Tissue density
 Location of cerebrospinal fluid spaces
Location of cerebrospinal fluid spaces
§ Ventricles
§ Virchow-Robin spaces
§ Sulci
• Drug administration
 Flow rate
Flow rate
§ Presence of air bubbles
TransMID (Transferrin-CRM107)
The first CED clinical study was of TransMID-107 (Xenova Group, Berkshire, United Kingdom), which is a thioether conjugation of human transferrin and a mutant form of diphtheria toxin known as CRM-107.18 TransMID targets tumor cells by binding to the transferrin receptor, which is overexpressed on rapidly dividing cells. In a phase 2 multicenter, open-label, single-arm study, 44 patients received intratumoral CED of TransMID at 0.67 ng/mL.18 The results confirmed the safety and tumor response data from the early studies. A phase 3 multicenter, randomized study in recurrent, nonresectable glioblastoma multiforme (GBM) is currently open with the best available standard treatment as the control arm.
NBI-3001 (IL4-PE)
IL4-PE (NBI-3001, Neurocrine, San Diego, California) is another recombinant toxin composed of interleukin-4 (IL-4) and a truncated form of the Pseudomonas exotoxin.19 A phase 1 study of intratumoral CED of IL4-PE started at a concentration of 2 ng/mL and was dose escalated to determine the maximum tolerated dose.20 Drug-related grade 3 or 4 CNS toxicity was seen in a total of 39% of patients in all groups, and no systemic toxicity was seen. A phase 2, multicenter, randomized study of intratumoral IL4-PE followed by tumor resection between 2 and 7 days after the completion of toxin infusion enrolled a total of 30 adult patients. The accrual was completed in 2003, but no final published results of the study were made available. There are no plans for a phase 3 study.
TP38
TP38 (TEVA, Inc., PA) is a recombinant toxin composed of transforming growth factor-α, a native epidermal growth factor receptor (EGFR) ligand, and a 38-kd fragment of the Pseudomonas exotoxin. TP38 binds to EGFR, and, once internalized, enzymatically arrests protein synthesis. A phase 1 study of intratumoral and peritumoral infusion of TP38 was performed in patients with recurrent malignant glioma with a concentration escalation of 0.025 to 0.1 µg/mL.21 Two catheters were initially placed during tumor resection, and then a total volume of 40 mL was infused. The TP38 was well tolerated and a maximum tolerated dose was not established. At the completion of the study, four patients had no recurrence of tumor at 55 to 116 weeks after treatment. The overall median survival for all patients after treatment was 23 weeks.
Cintredekin Besudotox (IL13-PE38)
Cintredekin besudotox is a recombinant toxin consisting of human interleukin-13 (IL-13) with the same 38-kd fragment of the Pseudomonas exotoxin. IL-13 receptors have been found in more than 90% of glioblastoma cells in vitro and in situ, whereas expression in the brain is not present or expressed at low levels.22 Intratumoral and peritumoral CED of IL13-PE38 has been investigated with four separate phase 1 studies. In the largest peritumoral phase 1 study, a maximum tolerated concentration of 0.5 µg/mL was observed.23 In this four-stage study, histological efficacy, maximum tolerated concentration, and maximum infusion time were assessed. The final stage explored the stereotactic placement of catheters after the tumor resection to improve the targeting of catheters into the peritumoral brain tissue. With the implementation of steroid guidelines, all patients tolerated the volume of infusion of 40 mL in two to three catheters. The median survival of all patients treated with peritumoral CED of IL13-PE38 (n = 44) was 44 weeks.24 Catheter placement was noted to be variable in the early portion of the study, with some catheter tips placed in the ventricle or other CSF spaces.
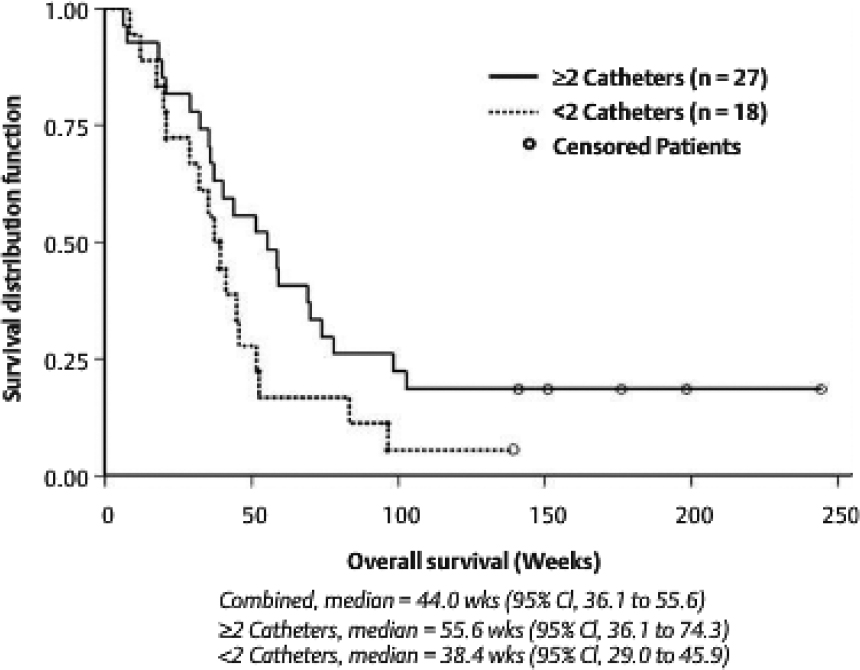
A retrospective review of catheter placement demonstrated a correlation with survival when at least two or more catheters had been placed optimally to minimize loss of drug into the CSF compartment. The 27 GBM patients with two or more optimally placed catheters had a median survival of 55.6 weeks, with 18.5% of patients surviving beyond 2 years after a single treatment (Fig. 20.3). There were no grade 3 or 4 adverse events associated with drug infusion at concentrations less than 0.5 µg/mL, and no systemic toxicities were observed. Delayed radiographic changes were observed in some patients 2 to 4 months after therapy, which responded to steroids and may represent an inflammatory response or nonspecific activity.25
A phase 3 multicenter, randomized study (known as the PRECISE study) was initiated in patients with first-time recurrent GBM. The patients were randomized 2:1 to surgery followed by peritumoral infusion of IL13-PE38 versus surgery and Gliadel wafer (MGI Pharma, Inc., Bloomington, MN) implant; 296 patients were enrolled from 52 centers, and there was no difference in survival and the adverse events profile between the Cintredekin besudotox group and the Gliadel wafer group (p = 0.31), with the exception that pulmonary embolism was higher in the Cintredekin besudotox group.24 In a separate study of these same patients, there was no difference in outcome in respect to catheter positioning.26
Fig. 20.3 Kaplan-Meier estimates for recurrent glioblastoma multiforme patients by catheter positioning in three phase 1 studies. CI, confidence interval.
• Although some studies have shown optimal catheter placement to be an important variable in the delivery of drug via CED, other studies have demonstrated that catheter positioning did not significantly impact patient outcome.
Future Directions
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree