11 Intraoperative Magnetic Resonance Imaging
Intraoperative magnetic resonance imaging (iMRI) first appeared in the neurosurgical operating room in the mid-1990s.1 Initial designs were admittedly cumbersome, as the surgeon was forced to operate within the magnet itself. Such constrictions limited a surgeon’s movements as well as the feasibility of additional intraoperative adjuncts, like the operating microscope. Image resolution was an added concern, as early models incorporated low-field-strength magnets such as the 0.2-tesla (T) magnet.
Such limitations hampered the widespread adoption of iMRI devices. However, new innovations such as ceilingmounted units and high-field-strength magnets of 1.5 to 3 T strength are expanding iMRI’s utility and efficiency (Table 11.1). As “next generation” iMRI suites emerge, the advantages of iMRI-guided craniotomies are increasingly recognized. Although iMRI has aided the surgical treatment of nonneoplastic disease,2 by far the greatest benefits of this intraoperative aid have been witnessed in the field of neuro-oncology.
 Extent of Resection
Extent of Resection
Maximal safe resection is a frequently, although not universally, accepted operative goal for many types of brain tumors. The primary benefit to iMRI-assisted surgery is the ability to identify residual pathological tissue in near real-time (Fig. 11.1). Such identification permits maximal resection of the lesion in question during the initial procedure. Maximal resections are thus achieved in addition to fewer secondary surgeries for residual disease.
Growing evidence, although mostly class 3, emphasizes the importance of extent of resection for both high- and low-grade glial tumors (Fig. 11.2). Critics often cite the absence of class 1 evidence (Oxford Centre of Evidence-Based Medicine, http://www.cebm.net/index.aspx?o=1025) in the neurosurgical literature to prove a survival advantage for greater surgical resection. However, one must also acknowledge the logistical and ethical limitations that likely preclude any future trial that randomizes solely on the basis of extent of resection. Would the neurosurgical community be willing to randomize an otherwise healthy 20-year-old patient with a suspected high-grade glioma of the right frontal pole to the biopsy arm of a surgical trial? Given these limitations, we are forced to rely upon well-controlled observational studies.
Controversy
• Observational studies suggest a correlation between extent of resection of gliomas and survival. However, randomized controlled studies, that is, class 1 evidence, do not exist to prove this view.
In 2001, a retrospective series of 416 histology-proven glioblastomas was published in which a 4.2-month overall survival advantage was found for patients who received at least a 98% extent of resection.3 Interestingly, this study has been referenced by both sides of the extent of resection debate, as it contributed to the so-called all-or-nothing principle. Proponents of maximal resection note the statistically significant benefit for the greater extent of resection. Opponents emphasize that essentially only complete resections led to a survival advantage, which in turn lessened the study’s clinical importance and relevance because a significant percentage of glioblastomas are not amenable to complete resection at presentation. Such lesions were frequently relegated to biopsy alone. Here, the notion of a maximal, albeit subtotal, resection is largely overlooked.
The only randomized clinical trial examining the extent of resection for high-grade gliomas randomized patients over 65 years of age with a suspected high-grade glial tumor to either stereotactic biopsy or craniotomy and resection.4 The median survival of the surgical group was double the median survival of the biopsy arm. Despite the prospective, randomized design, a number of limitations restricted the study’s findings, including a small sample size of only 30 patients as well as the inclusion of multiple pathologies.
The ALA-Glioma Study Group provided a unique opportunity to examine the effect of extent of resection on glioblastoma survival. The original study randomized patients to conventional microsurgery versus 5-aminolevulinic acid immunofluorescence-guided surgery.5 Although not randomized to extent of resection, the study did provide well-controlled, prospective data. A post-hoc analysis demonstrated a survival advantage for greater resection even after confounders like age and eloquence were identified and controlled.6 More recently, an extent of resection threshold for patients with newly diagnosed glioblastomas indicated that an extent of resection as low as 78% imparts a survival advantage.7
Table 11.1 Some Commonly Used iMRI Systems
| System | Company | Field-Strength (Tesla) |
| PoleStar® Surgical MRI | Medtronic, Inc. | 0.15 |
| VISIUS® Surgical Theatre | IMRIS, Inc. | 1.5–3 |
| Brainsuite® | Brainlab | 1.5–3 |
| AIRIS® | Hitachi Medical Systems America, Inc. | 0.3 |
| Signa SP® | General Electric Medical Systems | 0.5 |
| Ingenia® | Philips Medical Systems | 1.5–3 |
Pitfall
• Because of the strong feelings of surgeons and patients about the value of aggressive resection of gliomas, a proper randomized study can probably never be carried out to provide definitive proof.
A similar treatment paradigm emphasizing extent of resection should be considered in the management of low-grade glial tumors as well (Fig. 11.3). A series of 216 adults with low-grade hemispheric gliomas was reported in which improved outcome was predicted by a greater extent of resection.8 Patients who underwent at least a 90% extent of resection experienced a 5-year overall survival of 97%, whereas those patients who underwent less extensive surgery had a 5-year survival of only 76%. Such evidence argues in favor of the value of optimizing the extent of resection when treating both low- and high-grade gliomas, even when a complete resection is prohibited by the proximity of eloquent structures, as this surgical parameter likely contributes to improved patient outcome.
 Intraoperative Magnetic Resonance Imaging for Gliomas
Intraoperative Magnetic Resonance Imaging for Gliomas
Surgeons are notoriously poor judges of extent of resection during intracranial tumor resection. A recent study found that less than 25% of glioblastoma resections previously deemed “complete” by surgeon estimation were in fact confirmed by postoperative imaging.9 Intraoperative MRI provides a more objective means to gauge extent of resection at a time when further intervention remains possible.
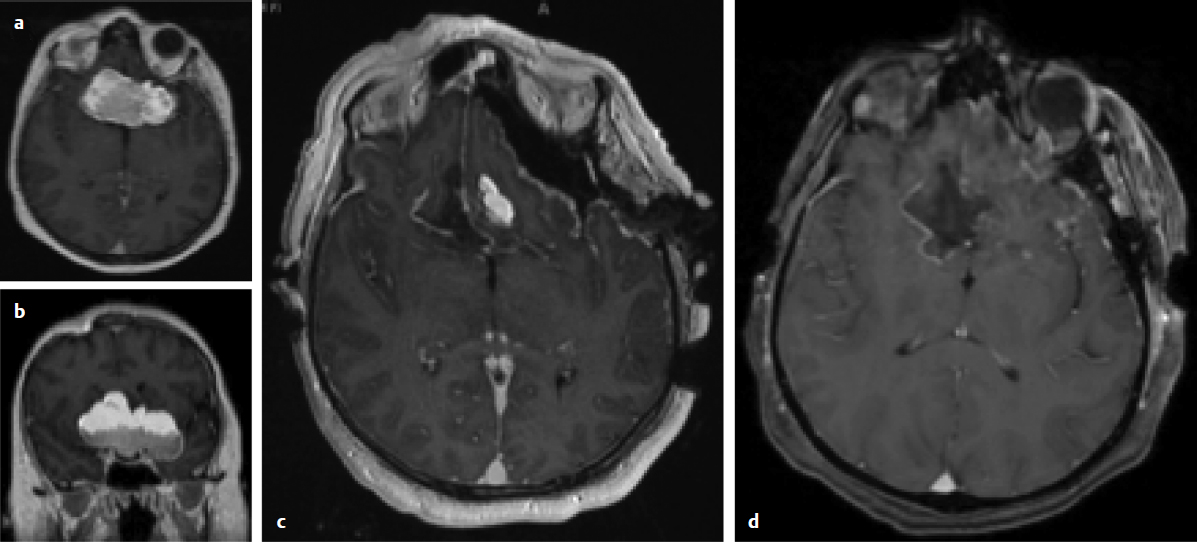
Fig. 11.1a–d Axial (a) and coronal (b) postcontrast, T1-weighted, preoperative magnetic resonance imaging (MRI) demonstrating a large teratoma of the anterior cranial fossa. (c) Intraoperative MRI demonstrating a small area of residual tumor. (d)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree