Benign germ cell tumors
Malignant germ cell tumors
Immature teratomaa
Germinoma
Mature teratoma
Embryonal carcinoma
Endodermal sinus tumor (yolk sac tumor)
Choriocarcinoma
Mixed germ cell tumor
Teratoma with malignant transformation
Intracranial GCTs most commonly arise from the pineal or suprasellar region – deep locations that have reduced the likelihood of gross total resection. For this reason, radiation alone, frequently encompassing a large treatment volume, was considered the preferred treatment standard. Over the last several decades, effective chemotherapy in combination with improved neurosurgical procedures and radiation techniques has resulted in dramatic improvements in survival. However, in children, the morbidity caused by radiation therapy, particularly with craniospinal irradiation (CSI), has led to de-escalation in volume and dose of radiotherapy while preserving high cure rates for patients with only focal disease (Shirato et al. 1997; Choi et al. 1998; Matsutani et al. 1998; Aoyama et al. 2002; Jensen et al. 2010).
In this chapter, we review the epidemiology of intracranial GCTs, the pathologic features of both benign and malignant GCTs, and their molecular and cytogenetic characteristics. We discuss the clinical features of intracranial GCTs and the role of imaging and laboratory investigations in diagnosis. In broaching the controversy surrounding diagnostic biopsy, we delineate the arguments for and against mandatory biopsy prior to treatment. We review recent changes in practice with de-escalation in radiotherapy and chemotherapy treatment approaches. Lastly, we discuss risk stratification to intensify treatment in patients with intracranial GCTs that have a poor prognosis.
6.2 Epidemiology
6.2.1 Location
Intracranial GCTs account for less than 4 % of pediatric brain tumors in North America, although slightly more common in Japan with an incidence of greater than 10 % among pediatric brain tumors. Most intracranial GCTs originate near the third ventricle, extending from the suprasellar cistern to the pineal gland. Pineal region GCTs outnumber those in the suprasellar region by a ratio of 2:1, but in 5–10 % of cases, the tumor is found in both regions (Jennings et al. 1985b). Whether this is due to synchronous bifocal disease or metastatic tumor, the spread remains unknown. Intracranial GCTs occur less commonly in other midline locations such as basal ganglia, thalamus, and ventricles, particularly the fourth ventricle. Intracranial GCTs have also been reported in the cerebellum (Nakase et al. 1994), brainstem (Nakajima et al. 2000; Madden et al. 2009; Hao et al. 2013), and optic nerves (Iizuka et al. 1996). By GCT subtype, germinomas are more frequent in the suprasellar region and in females, while NGGCTs are more common in the pineal region and in males.
6.2.2 Age, Sex, and Geographic Variation
In Western countries, intracranial GCTs account for 0.4–3.4 % of all intracranial tumors, whereas in Japan and Taiwan, intracranial GCTs are more common and account for 2.1–11.1 % of brain tumors (Jellinger 1973; Jennings et al. 1985b; Hoffman et al. 1991; Lin et al. 1997). This phenomenon is also seen in testicular GCTs for which the incidence in Japan is far greater than that seen in the United States (Packer et al. 2000). Most intracranial GCTs occur in adolescents and young adults (80–90 %), with peak incidence occurring at 10–14 years of age. However, these lesions can be seen in newborns as well as in older adults. In particular, NGGCTs preferentially arise in younger children, whereas germinomas are most common in teenagers (Jennings et al. 1985b; Rosenblum et al. 2007).
Intracranial GCTs are not distributed equally by gender. In the United States, between 1986 and 1995, incidence rates were 2.3 per million for males and 0.9 per million for females, representing a male predominance of 2.5:1. When examined by histology, NGGCTs demonstrate a male/female ratio of 3.2:1, while germinomas reveal a male:female ratio of only 1.8:1 (Jennings et al. 1985b). In females, 75 % of intracranial GCTs develop in the suprasellar region, whereas in males 70 % are found in the pineal area. The reason for these gender differences is unclear. Between the 1970s and the 1990s, the incidence of intracranial GCTs increased in the United States from 0.6 per million between 1975 and 1979 to 1.9 per million between 1990 and 1995 (Bernstein et al. 1999).
6.3 Pathology
6.3.1 Etiology
GCTs can be divided into extragonadal tumors and gonadal tumors, the latter encompassing half of all such tumors. Among extragonadal sites, half are sacrococcygeal and 40 % arise intracranially. Rare sites of extragonadal GCTs include midline regions such as the retroperitoneal and nasopharynx. Their sites of origin notwithstanding the features of GCTs, whether by light microscopy, electron microscopy, or enzyme or immunohistochemical assays, are identical (Jennings et al. 1985b; Felix and Becker 1990).
The pathogenesis of intracranial GCTs remains elusive. Although gonadotropins have been implicated in the pathogenesis of gonadal GCTs, such evidence for intracranial GCTs is lacking. One hypothesis is that GCTs arise most commonly near centers of gonadotropin regulation because such regions serve as sanctuary sites for undifferentiated germ cells (Jennings et al. 1985b). An additional role for the pineal gland in the neuroendocrine regulation of neoplastic growth has also been suggested (Lapin and Ebels 1981).
The etiology of intracranial GCTs is thought to be mismigration of primordial germ cells during embryonic development, followed by malignant transformation. According to the “germ cell theory,” primordial germ cells normally develop from the extraembryonic yolk sac endoderm and migrate to the gonadal folds. Germinomas as well as embryonal carcinomas can develop by further differentiation and malignant transformation of the original primordial germ cells. Embryonal carcinomas are composed of pluripotent cells that develop into endodermal sinus tumors, choriocarcinomas, or teratomas depending on the developmental pathway the cells undertake (Teilum 1976). Others have suggested that primordial germ cells can differentiate to yield either embryonal carcinomas or teratomas by differentiation through embryonic pathways or endodermal sinus tumors or choriocarcinomas by extraembryonic pathways (Takei and Pearl 1981).
The “germ cell theory” is supported by the fact that interaction of the c-kit receptor with its ligand, steel factor (SLF), mediates the migration of primordial germ cells. Lack of c-kit in animal models prevents germ cell migration. The gradient of SLF found from the yolk sac to the gonadal ridge is thought to guide the migration of primordial germ cells, and extragonadal GCTs are thought to arise from such mismigration. The proto-oncogene c–kit encodes a cell-surface receptor that carries an intrinsic tyrosine kinase activity in its cytoplasmic portion. The interaction of Kit with SLF leads to receptor dimerization, kinase activation, and tyrosine phosphorylation of specific cytoplasmic proteins. Mutations in Kit and SLF that result in a defective signaling pathway leading to infertility have been identified (Loveland and Schlatt 1997; Cushing et al. 2002).
An alternative theory, the “embryonic cell theory,” suggests that a pluripotent embryonic cell escapes normal developmental signals and gives rise to GCTs. This theory may be supported by the finding of altered Wnt pathway signaling components including distinct expression levels of E-cadherin and beta-catenin in various GCTs (Honecker et al. 2004; Snow et al. 2009). A third hypothesis contends that germinoma is the only neoplasm arising from germ cells, and other GCTs arise from misfolding and misplacement of embryonic cells into the lateral mesoderm early in embryogenesis, leading to the entrapment of these cells into a variety of different brain regions (Sano et al. 1989). And finally, a more recent hypothesis argues that neural stem cells, because of their pluripotent potential in vitro, may be the initiating cell for intracranial germ cell lesions (Hoei-Hansen et al. 2006; Tan and Scotting 2013).
6.3.2 Classification
The current World Health Organization (WHO) classification of GCTs is based on histology and tumor markers such as alpha-fetoprotein (AFP) and beta-human chorionic gonadotropin (β-HCG) that have become important in diagnosis as well as prognosis (Louis et al. 2007). As mentioned above, different GCTs may represent the malignant forms of distinct stages of normal embryonic development. For example, primordial germ cells result in germinomas, embryonic differentiation gives rise to teratomas and embryonal carcinomas, and extraembryonic derivatives of the yolk sac and trophoblast give rise to endodermal sinus tumors and choriocarcinomas, respectively. Intracranial GCTs can also be classified based on tumor markers found in serum or cerebrospinal fluid (CSF), which can influence diagnoses and prognoses of patients with intracranial GCTs. Typically, germinomas are nonsecreting tumors, whereas NGGCTs usually secrete AFP and/or β-HCG. Germinomas are associated with better prognoses than NGGCTs.
6.3.3 Histopathology
Intracranial germinomas are histologically identical to dysgerminomas of the ovary and seminomas of the testis (Beeley et al. 1973). Microscopically, they are large monomorphic cells with abundant clear cytoplasm, arranged in nests separated by bands of connective tissue. The differential diagnosis includes lymphoma and endodermal sinus tumor. Germinomas can be identified by either positive placental alkaline phosphatase (PLAP) staining or positive OCT4 staining, with the latter being increasingly adopted as superior, whereas endodermal sinus tumors stain positive for AFP (Hattab et al. 2005).
Among NGGCTs, teratomas are designated as mature or immature, based on the absence or presence of differentiated tissues. Mature teratomas contain mature tissues from all three embryonic layers (ectoderm, mesoderm, and endoderm). Immature teratomas are distinguished from mature teratomas by the presence of immature tissues, usually neuroepithelium. Embryonal carcinomas arise from pluripotent embryonic cells and are characterized by large cells with large nuclei and nucleoli with varying amounts of central necrosis. Embryonal carcinomas can produce both AFP and β-HCG. Unlike other GCTs, CD30 (Ki-1 antigen) immunohistochemical staining is positive in embryonal carcinomas. Endodermal sinus tumors arise from differentiated extraembryonic tissue, which usually occur as part of mixed GCTs, and produce AFP. Choriocarcinomas arise from placental trophoblastic tissue, which also generally occur as part of mixed GCTs, and are characterized by the presence of syncytiotrophoblasts that secrete β-HCG (Felix and Becker 1990; Hawkins 1990; Cushing et al. 2002).
6.3.4 Molecular Biology and Cytogenetics
Multiple complex karyotypes have been reported for intracranial GCTs including loss of chromosomes 4, 9p, 11, 13, and 17p as well as gain of chromosomes 8q, 21, and 1q. In addition, whereas isochromosome 12p seems to be important in the development of testicular tumors, its role in intracranial germ cell tumors is unclear. Multiple early studies observed low incidence in extragonadal tumors (de Bruin et al. 1994; Yu et al. 1995; Lemos et al. 1998), but isochromosome 12p has been found at more modest levels of 25 % in a more recent series (Sukov et al. 2010 A study of comparative genomic hybridization to analyze pineal region GCTs reported various abnormalities including gains on 12p (40 %), 8q (27 %), and 1q (20 %), as well as losses on 13q (47 %), 18q (33 %), 9q, and 11q (20 % each). The authors also noted different cytogenetic abnormalities based on histology. For example, the most common chromosomal changes in germinomas were −13q and −18q (38 % each), whereas in mixed teratomas, germinomas frequent abnormalities included +8q (100 %), +12p (75 %), −13q (75 %), and −9q (50 %) (Rickert et al. 2000). A recent series confirmed high-frequency (46 %) 12p polysomy (Sukov et al. 2010). Okada et al. examined 25 intracranial GCTs and found an increased number of X chromosomes in 23/25 cases and noted hypomethylation of the additional X chromosome in 81 % of the tumors. Only 20 % of cases had increased copy number of 12p and 12 % had loss of 13q. They concluded that along with the increased incidence of intracranial GCTs in males as well as predisposition in patients with Klinefelter syndrome, sex chromosome aberrations might have an important role in the development of GCTs (Okada et al. 2002).
In addition to cytogenetic changes, some of the genes that may be important in the development of GCTs have been defined. Alterations in the mdm–2 gene, often amplified in sarcomas, have been implicated in tumorigenesis of some testicular and intracranial GCTs. The mdm-2 is a negative regulator of the p53 tumor suppressor gene product and is, in turn, induced by p53. Iwato et al. searched for p53 mutations and mdm–2 amplifications in intracranial GCTs and found mdm–2 amplifications in 19 % of intracranial GCTs. Theoretically, increases in mdm-2 protein level would antagonize p53 function (Iwato et al. 2000b).
Iwato et al. examined the INK4a/ARF locus for alterations in intracranial GCTs and found alterations in 71 % of 21 tumors. The INK4a/ARF genes are tumor suppressor genes, and the INK4a protein inhibits cyclin-dependent kinases and decreases phosphorylation of the retinoblastoma protein, resulting in cell cycle arrest. The ARF protein interacts with mdm-2 and stimulates the latter’s degradation. Interestingly, alterations in INK4a/ARF were more common in germinomas (90 %) than in NGGCTs (55 %) (Iwato et al. 2000a).
A recent next-generation sequencing analysis of 62 intracranial GCT demonstrated frequent (53 %) novel somatic mutations in the KIT/RAS signaling pathway including KIT, KRAS, NRAS, CBL, and AKT1 (Wang et al. 2014). KIT mutations and overexpression were principally observed in germinomas, predominantly clustered in exons 17 and 11. Copy number gains were noted in AKT1 at 14q32.33 in 19 % of patients, the majority of which had wild-type KIT, KRAS, and NRAS. Loss-of-function mutations and loss of heterozygosity were noted in the tumor suppressors BCORL1 and CBL, respectively.
6.4 Clinical Features: Signs and Symptoms
Presenting symptoms of pineal region tumors are directly related to tumor location. Pineal region tumors usually present with symptoms of eye-movement disorders or symptoms caused by increased intracranial pressure due to obstructive hydrocephalus. Headache, nausea, and vomiting are the most common symptoms, seen in 56–93 % of patients. Blurred vision and somnolence are seen in 20–54 % of patients, while ataxia, seizures, and behavioral disturbances are seen in 10–28 % of patients (Saitoh et al. 1991; Drummond and Rosenfeld 1999; Steinbok and Cochrane 2001). Involvement of adjacent midbrain structures can result in visual disturbances, such as Parinaud’s syndrome, which are seen in 25–50 % of pineal region GCTs. Parinaud’s syndrome is an impairment of upward gaze in combination with dilated pupils that are nonreactive to light (pseudo-Argyll Robertson pupils), but responsive to accommodation. Upward gaze may in addition elicit rhythmic convergence of the eyes followed by retraction nystagmus of the eyes into the orbits. Eyelid retraction and conjugate downward gaze in the primary position (sun-setting sign) may be observed (Jennings et al. 1985b).
On examination, papilledema is present in about half of the patients. In patients with pineal region GCTs, approximately 80 % present with symptoms of increased intracranial pressure, whereas less than 10 % of patients with suprasellar GCTs present with increased intracranial pressure. Endocrinopathies such as diabetes insipidus or precocious puberty occur in patients with intracranial GCTs and account for approximately 6–12 % of presenting symptoms. In fact, patients with suprasellar GCTs most commonly present with endocrinopathies such as diabetes insipidus and manifestations of anterior pituitary dysfunction such as growth failure. These symptoms were seen in 87 % of patients versus only 8 % of patients with pineal region GCT (Jooma and Kendall 1983; Edwards et al. 1988; Hoffman et al. 1991; Saitoh et al. 1991; Kang et al. 1998; Steinbok and Cochrane 2001). Intracranial GCTs may infiltrate adjacent structures such as the hypothalamus (11 %) and third ventricle (22 %) or disseminate throughout the CSF (10 %). For endodermal sinus tumors and choriocarcinomas, dissemination is more common, and third ventricular involvement is present in over 40 % of cases. Extracranial spread to the lungs and bones has also been reported in approximately 3 % of patients (Gay et al. 1985; Jennings et al. 1985a, b).
6.5 Diagnosis and Staging
Operative morbidity and mortality prior to the 1980s were high and impeded histological diagnoses of many intracranial GCTs. Therefore, radiodiagnostic trials of 20 Gy historically functioned as surrogates for a histological diagnosis of germinoma, since these tumors were characteristically radioresponsive. Poor response to 20 Gy indicated an alternate diagnosis such as NGGCT or glioma. A robust response to 20 Gy suggested a diagnosis of germinoma, and treatment was continued to 50 Gy for definitive treatment. In light of the advances in neurosurgical techniques as well as the ability to differentially treat with chemotherapy, surgical biopsy in the modern era is generally much safer and usually recommended prior to treatment. However, some controversy remains whether biopsy is indicated for these tumors, and this decision determines the management plan.
Regardless of the ultimate histology, all GCTs are staged in a similar manner using magnetic resonance imaging (MRI) scans of the brain and spine in addition to CSF examination. Local lesions are categorized as M0. M1 disease is defined by microscopic dissemination in the CSF, while M2/M3 disease shows disseminated macroscopic lesions in the spinal region or cranial subarachnoid space visible on imaging.
6.5.1 Laboratory Investigations
AFP is normally expressed during embryonic development. It is the earliest serum-binding protein in the fetus, which reaches peak concentration at 12–14 weeks of gestation and then gradually falls to reach adult levels of 10 ng/dL at 1–2 years of age. As AFP levels decline during fetal development, albumin becomes the predominant binding protein. The presence of AFP (>25 ng/mL) indicates that there are malignant components in the tumor consisting of yolk sac elements or embryonal carcinoma. The half-life of AFP is 5–7 days and is a useful marker to follow, with one caveat: due to the variable rates of AFP levels in infants, AFP levels are less informative in this very young age group. Of note is the phenomenon of increasing AFP levels due to chemotherapy-induced tumor lysis and not necessarily due to disease progression. β-HCG is produced by syncytiotrophoblasts during pregnancy to maintain the corpus luteum, and minute amounts are found in normal adults. Pathologic elevations of β-HCG (>50 IU/L) are found when there is a clonal disorder of syncytiotrophoblasts, such as in choriocarcinoma, or when syncytiotrophoblastic giant cells are found in germinomas or embryonal carcinomas. Therefore, when an elevation of one of these tumor markers is present, it is highly suggestive of GCT.
Embryonal carcinomas secrete both AFP and β-HCG, while endodermal sinus tumors secrete only AFP, and choriocarcinomas secrete only β-HCG. However, in as many as 30 % of GCTs, more than one histological subtype is found (Matsutani et al. 1997). The most useful laboratory values for the diagnosis of GCTs are elevations of AFP and/or β-HCG in serum or CSF. It is important to sample both serum and CSF, as serum levels can be normal in the presence of elevated CSF levels and vice versa. If present, the protein levels can serve as useful tumor markers since they decrease as tumor burden decreases. GCTs that have elevations of these tumor markers show worse prognosis when matched with patients with identical histological diagnoses, but normal marker levels (Itoyama et al. 1995; Nishizaki et al. 2001). AFP can be used as a tumor marker in endodermal sinus tumors, and β-HCG is useful in choriocarcinoma.
Another helpful tumor marker is PLAP, which is a fetal isoenzyme of alkaline phosphatase, and is almost always elevated in germinomas (Cushing et al. 2002). Therefore, one controversial option in patients with elevated PLAP, but normal β-HCG and AFP, would be to assume the diagnosis is germinoma and treat accordingly (Steinbok 2001) (Table 6.2). However, PLAP is not readily available as a test in many institutions, and such empiric diagnoses are extraordinarily rare. Another marker for germinoma that has been employed is c-kit (CD117), and its soluble isoform, s-kit. Elevations of s-kit were found in the CSF of patients with germinoma and mixed GCT and may correlate with patients’ clinical courses. Moreover, the level of s-kit was remarkably higher in patients with tumor dissemination, such that s-kit has been a useful tumor marker (Miyanohara et al. 2002; Kamakura et al. 2006). In addition, immunohistochemical staining for OCT4, an 18-kDa POU-domain transcription factor encoded by the POU5F1 gene, has been shown to be a highly specific and sensitive test for germinomas, preferred over PLAP (Hattab et al. 2005). Recently, the novel stem-cell marker SALL4, a zinc-finger transcription factor upstream of OCT4, has been shown a highly sensitive and specific diagnostic marker for intracranial GCT expressed in germinomas, yolk sac tumors, and embryonal carcinomas (Mei et al. 2009). Glypican 3 (GPC3) immunostaining may also have diagnostic utility for detection of yolk sac tumors (Zynger et al. 2008).
Table 6.2
GCTs according to tumor markers
Tumor type | AFP | β-HCG | PLAP | OCT4 | SALL4 |
|---|---|---|---|---|---|
Mature teratoma | − | − | − | ± | ± |
Immature teratoma | ± | ± | − | ± | ± |
Pure germinoma | − | − | + | + | + |
Endodermal sinus tumor | + | − | − | – | + |
Choriocarcinoma | − | + | − | – | ± |
Embryonal carcinoma | + | + | − | ± | + |
Mixed germ cell tumor | ± | ± | ± | ± | + |
6.5.2 Diagnostic Imaging
Like other brain tumors, computed tomography (CT) and MRI are the most common modalities used to diagnose intracranial GCTs. Of historic interest only, pineal region tumors can be detected on plain skull films by the presence of calcifications. MRI is the study of choice, although CT has an advantage over MRI in identifying calcifications. The identification of calcification in the pineal gland in a child younger than 6 years old is an indication for an MRI, even when no mass is apparent on CT (Zimmerman and Bilaniuk 1982; Steinbok 2001).
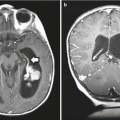
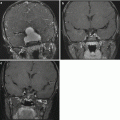
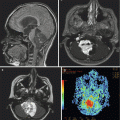
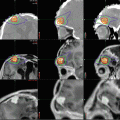
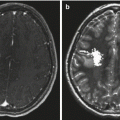
Findings on CT or MRI are almost never sufficient for the diagnosis of GCTs (Awa et al. 2014). Germinomas are usually diffusely enhancing on CT and MRI, whereas NGGCTs are more likely to be heterogeneous in part due to hemorrhage. A tumor in the suprasellar region in association with a pineal tumor is usually a GCT, most likely a germinoma (Fig. 6.1). Larger germinomas can have a heterogeneous appearance and fill the third ventricle (Fig. 6.2). A bifocal location is not guaranteed to be a germinoma as GCTs with mixed elements can also appear in two locations (Fig. 6.3). A recent or old hemorrhage seen in the tumor suggests an NGGCT, particularly common with choriocarcinoma (Fig. 6.4). Intracranial teratomas tend to be well circumscribed and have large cysts and calcifications within the tumor, which can be helpful in distinguishing them from germinomas (Fig. 6.5). Immature teratomas tend to have fewer cysts and calcifications and may secrete tumor markers (Fujimaki et al. 1994).
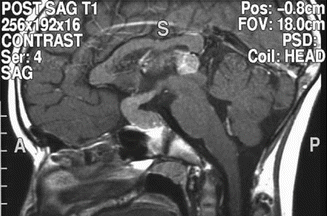
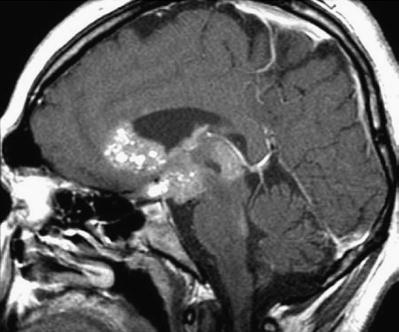
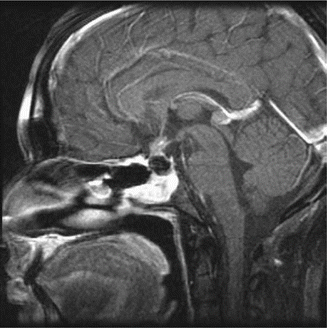
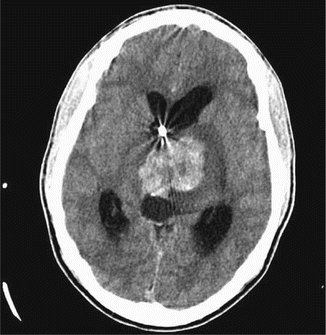
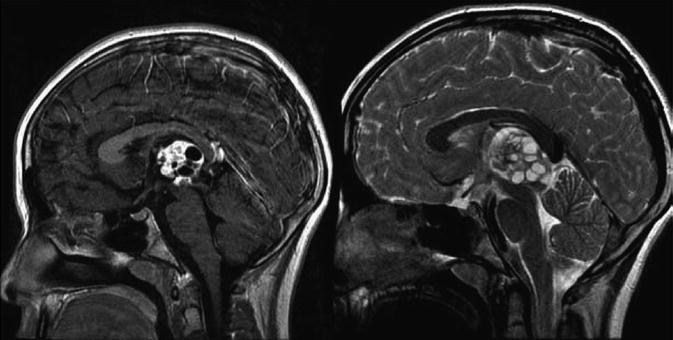

Fig. 6.1
A sagittal T1-weighted MR image following contrast administration shows an anterior third ventricle mass and a pineal region mass that both enhance relative to the normal brain. After biopsy, this tumor was diagnosed as a germinoma

Fig. 6.2
A sagittal T2-weighted image shows an extensive heterogeneous suprasellar germinoma with multiple cysts. The tumor fills the anterior portion of the third ventricle and extends posteriorly into the pineal region

Fig. 6.3
A sagittal T1-weighted image following contrast shows an unusual case of a mixed germ cell tumor containing both germinoma and teratoma located in the suprasellar, sellar, and pineal regions. The sellar component was biopsied through a transsphenoidal approach. The area of low signal intensity within the sella represents a fat patch to prevent postoperative CSF leak

Fig. 6.4
An axial CT image from a teenage boy who presented with headaches and a change in his mental status. A large partially hemorrhagic tumor is seen extending into the lateral ventricles. An endoscopic biopsy was consistent with choriocarcinoma. β-HCG as measured in the CSF was 88,767 IU/L (normal: <1.5). Hydrocephalus is present, and an external ventricular catheter has been inserted into the anterior portion of the ventricle

Fig. 6.5
A sagittal T1-weighted image following contrast (left) and a T2-weighted image (right) demonstrate the typical appearance of a mixed GCT, in this case a teratoma with germinoma. The tumor is heterogeneous with robust enhancement of the solid component although there are also multiple cysts within the tumor
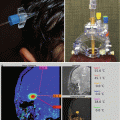
Molecular imaging approaches including positron emission tomography (PET) have been evaluated with initial reports of (11)C-methionine demonstrating greater diagnostic and treatment planning utility over (18)F-fluorodeoxyglucose (Okochi et al. 2014); however, PET approaches are not currently widely employed in diagnosis of intracranial GCT.
In addition to GCT, the differential diagnosis of a pineal lesion includes pineoblastoma, trilateral retinoblastoma in a patient with bilateral retinoblastoma, pineocytoma, glioma, meningioma, lymphoma, or a benign lesion such as a cyst. Benign cysts can generally be distinguished from malignant cystic neoplasms by the lack of enhancement or a very thin rim of enhancement surrounding a hypointense center (Steinbok 2001).
6.5.3 Obtaining Tissue Diagnosis
There is geographic variation in management strategies. In 1992, Oi and Matsumoto noted that the majority (84 %) of Japanese neurosurgeons were comfortable using a radiodiagnostic trial of 20 Gy in lieu of histological confirmation of a germinoma. In contrast, the majority (78 %) in Western countries recommended histological diagnosis as the initial management of pineal region tumors. This discrepancy may be due to the fact that pineal region tumors are much more common in Japan, and the incidence of germinomas in particular is higher (Oi and Matsumoto 1992). A follow-up study showed that by 1998, radical resection of the tumor was recommended as the initial procedure by only 22 % of Japanese neurosurgeons, while 39 % recommended biopsy, and 39 % recommended radiation therapy. The authors suggested tissue diagnosis by ventriculoscopic or stereotactic approach as the most appropriate initial step for the treatment planning of pineal region tumors (Oi et al. 1998).
Because histology influences the choice of treatment and is coincident with improved surgical techniques, the need for obtaining tumor histology is now recognized (Aydin et al. 1992; Sawamura et al. 1997). Although a definitive conclusion cannot be reached, a prudent strategy would be to utilize a safe and minimally invasive technique to obtain tissue for histological analysis. Such techniques would include endoscopic biopsy at the time of third ventriculostomy or aqueductoplasty or stereotactic biopsy. A review of 370 cases of stereotactic biopsies in France reported only 1.3 % mortality and 1 % major morbidity rates (Regis et al. 1996). Despite these relatively low rates, most surgeons are more comfortable with open biopsy in this region due to the close proximity of deep cerebral veins. One of the advantages of open biopsy is that sampling error can be minimized by taking several biopsies. This is particularly important since mixed GCTs are commonly encountered. Advances in surgical techniques allow open procedures to access the pineal region without major morbidity.
As results from intracranial GCT studies have emerged, stratification into “intermediate”- and “high”-risk prognostic groups has refined the role of biopsy. Specifically, serum and/or CSF β-HCG levels greater than 1000 IU/L and AFP levels greater than 1000 ng/mL nearly uniformly indicate the diagnosis of pure or predominant choriocarcinoma and yolk sac tumor, respectively (Matsutani et al. 1997; Kretschmar et al. 2007). Such conspicuous marker elevations may obviate the need for biopsy since they denote pure and/or predominant malignant elements and thus should be treated within the highest risk group. Current studies have further relaxed the need for biopsy in the presence of even lower serum or CSF levels of β-HCG or AFP >50 IU/L.
6.6 Treatment
6.6.1 Role of Surgery
Prior to 1970, surgery resulted in 25–70 % morbidity and mortality rates and led to radiotherapy becoming the treatment of choice with a modern 10-year overall survival, at least for germinomas, in the order of 80–100 %. In Japan, the standard of care was administration of a radiodiagnostic trial of 20 Gy followed by definitive doses of radiation if 20 Gy induced a tumor response (Handa and Yamashita 1981). Conventional radiotherapy for CNS germinomas involved 30 Gy of CSI followed by a boost to the primary disease site to a total dose of 50 Gy. The main role of surgery at that time was for treatment of hydrocephalus by placement of ventriculoperitoneal shunts, which resulted in peritoneal metastases at times (Brandes et al. 2000). Currently, other than as a diagnostic tool, surgery has no proven role in the treatment of intracranial germinomas due to the high operative morbidity and excellent outcomes with chemoradiotherapy (Pollack 2012). Several studies have shown no benefit to radical surgical resection in overall survival for germinoma patients (Sawamura et al. 1997).
For NGGCTs, surgery plays an important role along with other treatment modalities. In a study by Weiner et al., radical resection in addition to chemotherapy improved prognosis for patients with intracranial NGGCT. They recommended delayed surgical resection for patients who have normalized tumor markers with persistent radiographic abnormalities after three cycles of initial chemotherapy in order to avoid unnecessary radiation or further chemotherapy (Balmaceda et al. 1996; Weiner et al. 2002). For mature teratomas, it is generally accepted that gross total surgical resection is sufficient for cure. For immature teratomas, adjuvant chemoradiotherapy should be used if tumor markers are present because it is assumed that malignant germ cell elements are present. Even following gross total resection of nonsecreting immature teratomas, there is still a risk of relapse without additional adjuvant therapy (Sawamura et al. 1998b). Endoscopic approaches may more effectively access these midline suprasellar lesions (Somma et al. 2014; Tseng et al. 2012).
Second-look surgery, in particular, was proposed by some authors (Friedman et al. 2001; Weiner et al. 2002). A retrospective review of 126 patients enrolled on the First and Second International Central Nervous System Germ Cell Tumor Studies for patients with newly diagnosed CNS GCTs sought to establish the role of delayed surgical resection in patients who exhibit less than complete radiographic response despite declining tumor markers after initial chemotherapy. Indeed, after at least three cycles of chemotherapy, ten patients underwent delayed surgical resection due to residual radiographic abnormalities in the setting of declining or completely normalized serum and CSF levels of β-HCG and AFP. Second-look surgery revealed three mature teratomas, two immature teratomas, and five cases of necrosis or scar tissue alone. At an average follow-up time of 36.9 months (range 3–96 months), only three of the ten patients had experienced tumor recurrence. Three of the four patients with NGGCTs whose tumor markers had not completely normalized ultimately developed tumor dissemination/progression and required radiation therapy even though pathology at second-look surgery showed only teratoma or necrosis/scar tissue. In contrast, three of four patients with NGGCTs whose tumor marker levels had completely normalized did not progress and did not require radiation therapy. The authors concluded that delayed surgical resection was indicated in patients with GCTs who have residual radiographic abnormalities and normalized tumor markers following chemotherapy (Weiner et al. 2002). The current experimental schema for localized GCT, ACNS1123, recommends second-look surgery following induction chemotherapy for less than complete response with normalization of markers (COG ACNS 1123 2012).
6.6.2 Chemotherapy
Chemotherapy was incorporated into the treatment of intracranial GCTs after agents known to have activity against testicular GCTs were shown to cross the blood–brain barrier (Ginsberg et al. 1981; Brandes et al. 2000). As single agents, actinomycin D, vinblastine, bleomycin, doxorubicin, cisplatin, carboplatin, etoposide, ifosfamide, and cyclophosphamide are active against GCTs, and combinations of these agents are the basis for treatment regimens. The most common combinations are PEB (cisplatin, etoposide, and bleomycin), PVB (cisplatin, vinblastine, and bleomycin), and JEB (carboplatin with etoposide and bleomycin) (Hawkins et al. 1986; Pinkerton et al. 1990; Cushing et al. 2002; Einhorn and Donohue 2002). In addition, ifosfamide has been found to be the third most active agent against GCTs, following cisplatin and etoposide, and was investigated as salvage therapy in patients with refractory disease (Nichols 1996). The recent Children’s Oncology Group NGGCT study, ACNS0122, utilized a regimen of carboplatin and etoposide alternating with ifosfamide and etoposide that demonstrated safety and efficacy.
However, efforts to omit radiation and treat intracranial GCTs with chemotherapy alone have been less promising. Yoshida et al. saw a response rate of 80–85 % in patients treated with a combination regimen of cisplatin and etoposide, but survival rates at 2 years were disappointing at 88 % in patients with germinomas and 48 % in patients with NGGCTs. Baranzelli et al. reported that of 13 AFP- and β-HCG-secreting GCTs treated by chemotherapy and surgery alone, 12 recurred. Approximately 50 % of the patients with tumor recurrence experienced remission following salvage radiation therapy. Because of the need for salvage radiation therapy, the authors concluded that focal radiation therapy should be part of the treatment of these tumors.
Finally, Balmaceda et al. enrolled 45 patients with germinomas and 26 with NGGCTs in a clinical study and treated them with four cycles of carboplatin, etoposide, and bleomycin. Those with a complete response defined by imaging studies received two additional cycles, and those with less than a complete response received two additional cycles intensified by cyclophosphamide. Overall, 78 % of patients achieved a complete response with chemotherapy only. However, of the 54 patients surviving at 2 years, 32 (59 %) received irradiation. The 2-year survival was 84 % for patients with germinoma and 62 % for those with NGGCT. Thus, it appears that chemotherapy alone does not provide comparable cure rates when compared to combined modality treatment (Yoshida et al. 1993; Balmaceda et al. 1996; Baranzelli et al. 1998). Of particular pertinence to NGGCTs, results from the First International CNS GCT Study Group indicated that approximately one third of NGGCT patients who initially had a complete response to chemotherapy had recurrent disease. And most importantly, unlike recurrent germinoma patients, these NGGCT patients were not amenable to salvage with radiation therapy (Balmaceda et al. 1996).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree