Intracellular Signaling and T Cell Activation
Learning Objectives
• Draw the immunologic synapse showing primary and secondary components
• Explain the function of adaptor proteins
• Discuss the advantages of T cell receptor (TCR) clustering
• Identify the three transcription factors necessary for interleukin 2 (IL-2) synthesis
• Identify the signaling pathway activated by CD28–B7 interactions
• Identify the signaling pathways activated by CD28–B7 engagement
• Compare the mechanisms by which cyclosporine and tacrolimus inhibit T cell signaling
• Recognize the mechanism by which sirolimus inhibits T cell signaling
• Compare and contrast the structure of high-affinity and low-affinity IL-2 receptors
• Identify the immunologic defect in X-linked severe combined immunodeficiency (SCID)
Key Terms
AP-1 complex
Autocrine signaling
Cyclosporine A
Fos
Immunologic synapse
Jun
NFAT
Nuclear paracrine factor-κB (NF-κB)
Phospholipase C
Phosphokinase C
Ras
Sirolimus
Tacrolimus
Introduction
An immune response requires the activation and proliferation of antigen-stimulated T cell clones. Surface interactions initiate intracellular signaling that results in the synthesis of numerous proinflammatory cytokines, survival factors, and growth factors. One of the most important growth factors is interleukin 2 (IL-2), a 15,500-kDal protein produced by activated T cells. IL-2 has both autocrine and paracrine functions. In autocrine signaling, activated T cells produce IL-2, which binds to receptors on the same cell to initiate T cell growth and proliferation. Soluble IL-2 can react with nearby activated T cells expressing IL-2 receptors (paracrine signaling) as well. IL-2 is also required for the survival and function of regulatory T cells that damper the response to self-antigens.
Signaling Pathways for Interleukin 2 Synthesis
Engagement of the TCR–HLA molecules and CD28–B7 molecules activates several signaling pathways, which culminate in the translocation of three nuclear transcription factors (NFAT, NF-κB, and an AP-1 complex consisting of Fos and Jun proteins). In the nucleus, transcription factors bind to the promoter regions of IL-2 genes, which begin the transcription of messenger ribonucleic acid (RNA) and the translation of IL-2 protein. The pathways involved in the synthesis of IL-2 are shown below.
Pathways Activated by TCR–HLA Interactions
Pathways Activated by CD28–B7 Interactions
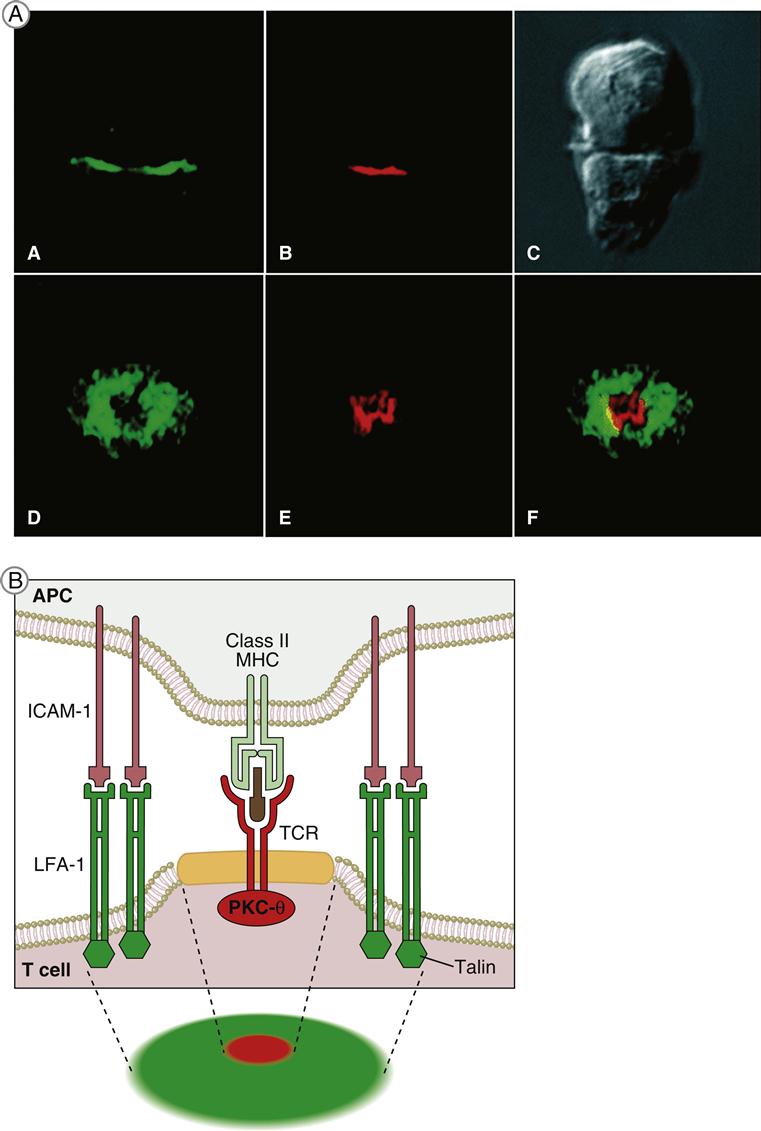
Immunologic Synapse
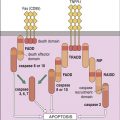
T cell receptor (TCR)–mediated signaling is initiated by a structure known as the immunologic synapse or the supramolecular activation cluster (SMAC). The synapse is a “bull’s eye–like” structure with the engaged TCR–HLA class I or II molecules and CD28–B7 molecules clustered in the center (Figure 7-1).
Molecular clustering serves three purposes: (1) TCRs engage antigen-loaded HLA molecules with an intermediate affinity. Although these complexes only survive for short periods, they can transduce an activation signal to the nucleus. However, successful activation of T cells requires serial and sustained engagement of TCR–HLA complexes. (2) In the clustered arrangement, multiple TCRs interact with small numbers of antigen-loaded HLA molecules on antigen-presenting cells (APCs). (3) Clustering also congregates multiple cytoplasmic immuno receptor tyrosine-based activation motifs (ITAMs) near adaptor proteins, which are necessary for downstream signaling. Following the engagement of CD4 or CD8 with invariant HLA molecule domains, leukocyte-specific protein tyrosine (lck kinase) is activated and phosphorylates ITAMs. Activated ITAMs serve as “docking stations” for adaptor proteins.
Adaptor Proteins
Adaptor proteins form short-lived complexes with other proteins to transduce membrane activation signals to the major cytoplasmic signaling pathways. The most studied adaptor protein is zeta (ζ)-chain associated protein of 70 kDal (Zap-70). Phosphorylation of two ITAMs on TCR ζ-molecules creates a “docking site” for ZAP-70. CD4-activated or CD8-activated lck phosphorylates ZAP-70, which becomes an active kinase. ZAP-70 phosphorylates phospholipase Cγ1 and another adaptor protein called linker for activation of T cells (LAT).
Phosphorylated LAT serves two functions: (1) It provides a “docking site” for growth factor receptor-bound protein 2 (Grb-2) and its associated protein son of sevenless (SOS). (2) The GrB-2–SOS complex activates Ras, the initiating signal in the MAPK pathway. LAT also activates phosphokinase Cγ1 (PKC), the initiating signal for the NF–κB pathway.
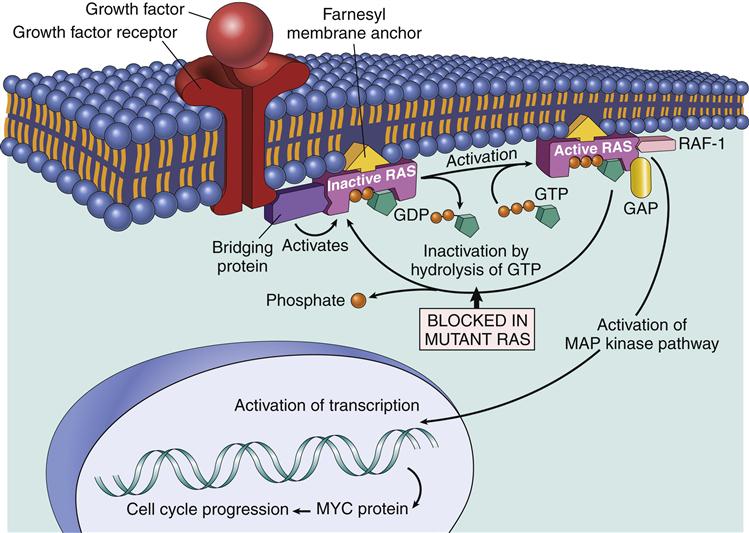
The Ras–Mapk Pathway
One component of the AP-1 transcription factor complex necessary for the synthesis of IL-2 is a product of the Ras–MAPK pathway. Rat sarcoma protein (Ras) is a small G protein, which is regulated by guanosine diphosphate (GDP) and guanosine triphosphate (GTP) in the cytoplasm. GTP activates the Ras protein. Hydrolysis of GTP and removal of a phosphate inactivates Ras (Figure 7-2). In T cell activation, Ras transduces signals from the surface receptor to the MAPK pathway. Hydrolysis of GTP is controlled by the presence or absence of Grb–SOS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree