CHAPTER 20 Intestinal Parasites
Intestinal Parasitosis at a Glance
Etiologic Agents of Intestinal Parasitosis
Intestinal parasitosis constitutes one of the most common types of human infections. One-third of the world’s population is affected by these organisms, including people living in both tropical and temperate climates, and it has been shown that all immigrant groups are affected to some degree.1 These infections often go unnoticed, but have the potential to cause serious health consequences. Therefore, familiarity with intestinal parasites is important for any healthcare provider caring for immigrant populations.
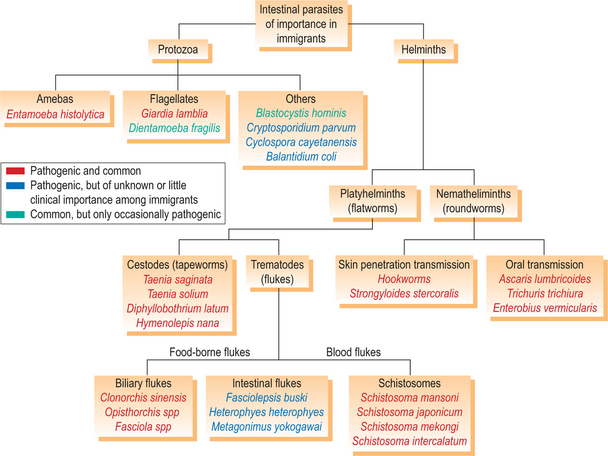
Parasites are broadly categorized as single-celled protozoa and multicellular helminths, or worms (Fig. 20.1). Both types frequently infect the human gastrointestinal system. The helminths consist of two phyla: the hermaphroditic platyhelminthes (flatworms) and the nemahelminthes (nematodes or roundworms), which consist of separate male and female worms. The platyhelminthes are further subdivided into two classes: the trematodes (flukes) and cestodes (tapeworms).
Nematodes are the parasites that most closely resemble common soil worms. Intestinal nematodes are transmitted to humans via two primary mechanisms: ingestion of soil contaminated with infective eggs (Ascaris lumbricoides, Trichuris trichiura) or by penetration of skin with infective larvae (hookworms, Strongyloides stercoralis). Ascaris, Trichuris, hookworms, and Strongyloides are all considered geohelminths, meaning at least part of their lifecy-cle takes place in the soil. Strongyloides is unique in that an alternative soil-independent cycle can occur in which affected individuals are repetitively autoinfected with infectious larvae. Consequently, immigrants may have Strongyloides infections for many years after leaving endemic areas. Enterobius vermicularis, pinworm, is a common intestinal nematode throughout the world, spread by fecal–oral transmission and does not involve a soil stage. Important nonintestinal nematodes include the tissue-dwelling filariae, are discussed in Chapter 36. There are many nematodes which are unable to develop into adult worms within humans. However, larval stages of many of these organisms can cause considerable morbidity by eliciting inflammatory responses in a variety of tissues. Some of these nonhuman nematodes of potential importance among immigrants include Toxocara canis, Angiostrongylus species, Gnathostoma species, and Trichinella spiralis.
Trematodes are a group of parasites that can cause chronic infections with many important long-term consequences. All trematodes require intermediate snail hosts. The manner in which the infective larvae that exit the snail go on to enter humans defines two broad groups of trematodes, the blood flukes and the food-borne flukes. The blood flukes infect humans by direct skin penetration, while the food-borne flukes, fittingly, infect humans through the consumption of food. There are five blood flukes of human importance, all of which are part of the genus Schistosoma. For detailed information on schistosomiasis, see Chapter 39. Food-borne trematodes can be divided into the biliary liver flukes (Opisthorchis species, Clonorchis sinensis, and Fasciola species), intestinal flukes (Fasciolepsis buski, heterophyids, and echinostomes) and lung flukes (Paragonimus species). All food-borne trematodes are transmitted via consumption of raw fish, crustaceans, or aquatic plants that are contaminated with the infective stages.
The cestodes, or tapeworms, comprised of characteristic segments, or proglottides, are relatively benign. All, except the human dwarf tapeworm, are transmitted to humans through consumption of undercooked meats. When humans consume undercooked beef, pork, or fish with larvae-containing cysts, they become infected with the adult form of Taenia saginata, Taenia solium, and Diphyllobothrium latum, respectively. These three species can grow to impressive lengths within the intestines. If eggs, passed in the feces of individuals harboring an adult Taenia solium worm(s), are accidentally ingested by fecal–oral contact, humans become accidental hosts for the larval stages and cysticercosis may develop with potentially serious consequences, which are discussed in Chapter 27. A similar process, reviewed in Chapter 28, occurs if humans accidentally ingest eggs of the Echinococcus tapeworms of carnivorous animals. Hymenolepis nana, the dwarf tapeworm, is the most commonly detected human cestode in nearly all immigrant groups. This likely is due to its simple fecal–oral means of transmission and its ability to persist in a cycle of autoinfection.
The pathogenic protozoa most likely to affect immigrants include the amoeba, Entamoeba histolytica, and the flagellate, Giardia lamblia (also known as G. intestinalis or G. duodenalis). Although many other protozoa are known to cause intestinal disease throughout the world, such as Crypotosporidium parvum, Cyclospora cayetanensis, and Balantidium coli, the importance of these organisms in immigrant populations remains unclear. Most protozoa dete-cted in immigrants are considered nonpathogenic. However, two of these organisms, Blastocystis hominis and Dientamoeba fragilis, traditionally thought to be benign commensals, may actually cause disease in some individuals. Blastocystis hominis is the most commonly detected intestinal parasites in all refugee populations arriving to the US, affecting 20–40% of refugees from Africa, the Middle East, Southeast Asia, Eastern Europe, and Latin America.2 However, since the pathogenic potential of this protozoan is debated, it is typically not reported in surveys of intestinal parasite prevalence.
Nonpathogenic parasites
All intestinal helminths are potentially pathogenic. However, many protozoan parasites detected in screening stool ova and parasites (O&P) specimens are nonpathogenic (Table 20.1). These organisms should not be treated (except for symptomatic Blastocystis hominis and Dientamoeba fragilis infections). Further investigations are always indicated among symptomatic patients who have only nonpathogenic parasites detected. Their presence indicates time spent in areas where poor sanitation led to fecal–oral contact. As a group, these nonpathogenic protozoans account for the first or second most common type of intestinal parasite detected in immigrant populations, depending on whether one considers Blastocystis hominis to be a pathogen.2
Table 20.1 Nonpathogenic intestinal protozoa
| Never pathogenic | Occasionally pathogenic |
|---|---|
Adapted with permission from reference 69. © 2004 by the Infectious Diseases Society of America.
Epidemiology
Immigrants and refugees from every region of the world are at risk for harboring intestinal parasites. Although certain parasites are seen more often in those from tropical locations, even individuals from temperate climates are at risk. Except for Giardia, mandatory federal reporting of intestinal parasitic infections in the US was discontinued in 1994. Therefore, the primary sources of prevalence data for parasitic infections in immigrant groups in the US are individual state refugee screening programs and published series on parasitic infections in convenience samples of immigrants in specific areas.1 Although current information is lacking on the prevalence of intestinal parasites in nonrefugee immigrant groups, several studies in the past describe the patterns of intestinal parasitosis in these populations.
Overall prevalence
The overall reported prevalence rates of pathogenic intestinal parasites among refugees, immigrants, and migrant workers in North America have ranged between 8.4% and 86%.1,3–16 This wide range can be explained primarily by differences in the geographic origin and ages of the populations studied. Other determinants include education level and past occupational exposures. Additionally, for refugees, the implementation of pre-departure empiric treatment with albendazole in 1999 greatly altered the detection rates of certain parasites. Furthermore, methodological differences partially explain the wide range in reported prevalence. For example, intestinal parasitosis rates among a population of Cambodian refugees in New York varied from 31% to 86% depending on the method of stool examination.14
These past studies all utilized various methods of stool microscopy for ova and parasites (O&P) to determine prevalence. Relying on stool microscopy may underestimate the true prevalence of intestinal parasitic infections due to poor sensitivity for several organisms, especially Strongyloides.17 On the other hand, despite the low sensitivity of stool O&P examinations to detect Entamoeba histolytica, all past surveys overestimated the prevalence of E. histolytica infections due to the inability to differentiate this organism morphologically from the more common nonpathogenic Entamoeba dispar and Entamoeba moshkovskii.18
Geography
Country of origin is likely the strongest predictor of intestinal parasitosis.3 Refugees of all ages from Southeast Asia have consistently been shown to have very high rates of intestinal parasitosis. Among refugees from this region resettling in North America in the 1970s and 1980s, prevalence ranged from 37% to 78%.13 In more recent years, Southeast Asians have consistently been found to be the most highly affected refugees with 22–48% of individuals harboring pathogenic parasites. In the same studies, refugees from sub-Saharan Africa follow close behind with rates of 16–43%.4,5,19 Those from the Middle East and Eastern Europe carry slightly lower burdens of intestinal parasites, ranging 13–32% and 8–22%, respectively.5,19
These geographic variations are explained primarily by differing rates of helminthic infections. For example, compared to refugees from the Middle East and Eastern Europe, those from Africa and South Central Asia (including Afghanistan, Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan, and Sri Lanka) were 5.9 and 8.0 times more likely to have a helminthic infection, respectively. In contrast, there was no statistical difference in protozoan infections among refugees from these various regions.1 This study did not include refugees from Southeast Asia.
Very little current information is available for Latin American refugees and immigrants. In a group of Latin American refugees to Sweden, 42% harbored pathogenic parasites.19 Similar high levels were noted among Latin American refugee claimants within Montreal in 1987. In fact, among 1967 individuals originating from over 70 countries throughout the world, those from Central America had the highest odds of having an intestinal parasite in multivariate analysis (OR = 2.4, 95% CI 1.5–3.7).3 Rates of helminthic carriage were as high as 65% in claimants from some Central American countries. Older data on Latin American immigrants to the US from the late 1970s to early 1990s reveal rates of pathogenic parasite carriage of 35–46%.8–11
Age
In general, younger immigrants are more likely to harbor intestinal parasites than adults. Among 2129 refugees arriving in Minnesota in 1999, the prevalence of having at least one pathogenic parasite was 30% for those under 18 years of age, as compared to 15% among those 18 years of age or older (p < 0.001).4 School-aged children, aged 7–17 years old, were most heavily affected. Most protozoan pathogens disproportionately affect immigrants under 10 years old, while helminthic infections tend to affect older children and young adults most frequently.3 Young children actually have the lowest rates of helminthic infections of all age groups.3 The type of helminths detected also varies by age. For example, among Southeast Asian refugees, school-aged children had the highest rates of Trichuris, Strongyloides, and Hymenolepis nana infections, while adults had the highest prevalence of hookworm and Clonorchis infections.11,14
Age may, in fact, be the only known predictor of the likelihood of protozoan infections among immigrants and refugees. Among refugee claimants to Montreal, the frequency of protozoan infections significantly increased with decreasing age. Protozoan infections, but not helminthic infections, were noted to be significantly more common in newly arrived refugees to California under the age of 18 years (OR = 2.2, 95% CI 1.2–4.2).1 This age variation appears to be most likely explained by especially high rates of Giardia in children. Entamoeba histolytica is more evenly distributed by age.14,15
Education
Among adult immigrants, the amount of past formal education has been strongly associated with the likelihood of helminthic infections. Half of all adult refugee claimants in Montreal with less than 5 years of schooling were found to have helminthic infections. This compared to 32%, 24%, and 12% of immigrants with 5–9, 10–14, and 15 or more years of education, respectively (p < 0.0001).3 This is in agreement with recent information on refugees arriving in California, in which there was a trend for adult refugees with less than 12 years of education to be at increased risk for helminthic infections (OR = 2.2, 95% CI 0.93–5.1).1 In both studies, level of education did not affect the likelihood of protozoan infections.
Parental education levels likely affect their immigrant children’s likelihood of parasite carriage as well. Although this has never been reported for immigrants to North America, a study in Turkey revealed that children whose mothers have less than a primary school education were significantly more likely to harbor parasites.20
Occupation
Immigrants who formerly worked in agriculture or fishing in their countries of origin may be more likely to harbor a variety of helminths as a result of occupational exposures.21,22 Both farming and fishing may increase the likelihood of hookworm infection.22,23 Chronic trematode infections can result from work exposures and may cause disease many years after immigration. For example, farming has been shown to increase the likelihood of Schistosoma haematobium infections in West Africa and Schistosoma japonicum infections in China.24,25 Fishing increases the likelihood of Schistosoma mansoni infection among people in East Africa.26 Individuals who have worked closely with sheep or cattle may be at risk for infection with Fasciola species.27 Farming may also increase the risk of Strongyloides stercoralis infections.28
In addition to the occupational exposures in countries of origin, the types of work immigrants do after settling in their new countries may place them at increased risk of intestinal parasites. The prevalence of pathogenic parasites among migrant farm workers in the southeastern area of the US has been found to be very high. Even though workers born in Central America and Haiti have much higher rates of pathogenic parasite carriage compared to US-born workers, there is evidence to suggest that ongoing transmission occurs among all workers on farms.16
Gender
Some of the occupational exposures described above may lead to higher rates of infection in men. This was noted for hookworm and Schistosoma infections in Africa.22,24,26 Among refugee claimants in Montreal, hookworm infections were almost twice as common in men.3 However, considering all pathogens detected in refugees entering the US and Canada, males have just a slight, often statistically nonsignificant, increased likelihood of harboring intestinal parasites.1,3,4
Close contacts
Intestinal parasitosis in individual immigrants is more likely if family members have been found to harbor parasites. Among a group of Southeast Asian refugees, the percentage of families with more than one individual carrying a pathogen was 86%, while the overall prevalence in the study group was 61%.12
Past living circumstances
Those who have lived in areas destroyed by wars may be at risk for parasites that otherwise are nonendemic to certain regions. For example, high rates of hookworm infections have been reported in refugees from Bosnia, an area with a typically low hookworm risk.19 Also immigrants who have spent time in refugee or emigration camps are often more likely to harbor parasites than others from the same countries who have not lived in such camps.29,30 However, in the case of refugees, this observation has been affected by the advent of pre-departure albendazole therapy.
Pre-departure albendazole treatment
In 1997, the Centers for Disease Control and Prevention (CDC) and the International Organization for Migration conducted an enhanced screening for intestinal helminthic and protozoan infections in a group of Somali refugees living in refugee camps in Kenya. They found that 38% of those screened harbored pathogenic intestinal parasites and, therefore, decided to treat empirically all nonpregnant refugees over the age of 2 years with a single 600 mg dose of albendazole within 3 days prior to departure to the United States.31 In May of 1999, the CDC extended this practice to refugees from locations throughout sub-Saharan Africa. At some point after 1999, individuals in Southeast Asian refugee camps relocating to the US also began receiving this same treatment regimen prior to departure.
Pre-departure albendazole treatment has dramatically decreased the prevalence of parasitic infections detected in stool samples in newly arrived refugees.6,15 A reduction from 24% to 4% in the prevalence of intestinal helminth infections has been documented in African refugees arriving in Massachusetts before and after May 1999, respectively (OR = 0.15, 95% CI 0.09–0.24).15 In this study, those who arrived after the initiation of pre-departure treatment had over a 90% lower odds of harboring Ascaris (OR = 0.07, 95% CI 0.01–0.58), hookworm (OR = 0.03, 95% CI 0.00–0.29), or Trichuris (OR = 0.05, 95% CI 0.02–0.13) compared to those who arrived before 1999. Surprisingly, a decrease in Entamoeba histolytica/dispar prevalence was also detected (OR = 0.47, 95% CI 0.26–0.86).15
In Northern California, a relatively low overall pathogenic parasite prevalence of 14% was observed in a group of refugees arriving between 2001 and 2004, after implementation of universal predeparture treatment. However, the investigators noted that the group with the highest prevalence of parasites, sub-Saharan Africans, came from the region of the world where pre-departure treatment is most highly enforced. This is likely explained by the relatively higher rates of Strongyloides and Schistosoma in this group.1 Albendazole has no activity against Schistosoma and is not the most effective treatment for Strongyloides, especially when given as a single dose.
Types of intestinal parasites commonly detected in different locations
There is very little effect of geography on the prevalence of protozoan infections. Excluding Blastocystis hominis, Giardia is the most or nearly the most common pathogenic parasite detected in all newly arrived immigrant populations.1,5,7–9,15,19 It occasionally falls second behind Trichuris or hookworm infections. The other protozoan pathogens, including Entamoeba histolytica and Dientamoeba fragilis, also show little geographic variation.1
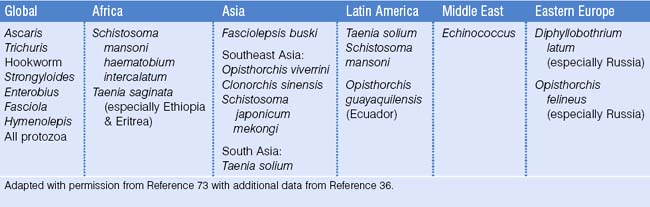
In contrast to protozoan infections, the epidemiology of helminthic infections varies more by region of origin. It is difficult to make broad generalizations regarding the types of parasites most frequently encountered by people in different areas of the world. There can be significant variation in the ecology of parasitic infections even within a relatively small region of the world. For example, large differences have been noted in both the overall prevalence of parasitic infection and types of parasites detected among Cambodian, Hmong, Laotian, and Vietnamese refugees.12 Nevertheless, it is important to have a general idea of which types of helminths are especially common in different populations. Geographic distributions of important intestinal parasites are listed in Table 20.2.
Clinical Manifestations
In general, most individuals harboring pathogenic intestinal parasites are asymptomatic at the time of screening. Most investigators have failed to find correlations between symptoms and the presence of intestinal parasitosis. In a study of East African immigrants living in Minnesota, the presence of abdominal pain, nausea, vomiting, indigestion, or diarrhea had a sensitivity of 71% and a specificity of 24% for detecting the presence of pathogenic intestinal parasites. These values translate into a positive predictive value of only 26% and a negative predictive value of 69%, indicating the inability to base clinical decision-making on the presence or absence of gastrointestinal symptoms.32 Other studies also have failed to find associations between gastrointestinal symptoms and intestinal parasites among Southeast Asian refugees33 and Latin American immigrants.8,9 The lack of correlation with symptoms, however, should not downplay the pathogenic potential of intestinal parasites. The following sections will review important clinical aspects of some of these organisms.
Nematodes
Etiology:
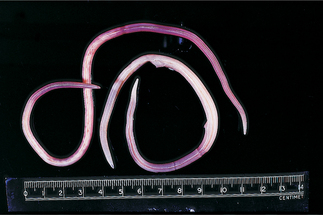
Ascaris lumbricoides is the largest intestinal nematode of humans, reaching lengths of 40 cm and diameters of 6 mm. The description of the worm in Box 20.1 is pathognomonic for ascariasis.
Box 20.1 Case study 1
An actual email: “I am an English Language Learners teacher and have many students from Liberia. I had a student complain about coughing up a long white worm as he was eating a lemon at lunch (Fig. 20.2). I sent him to the nurse at school, as he said this was the second time it had happened to him. She sent him back to class saying there wasn’t enough to tell anything at this point. Is there anything you can suggest, or anywhere I can direct his parents?”
Epidemiology:
Ascaris lumbricoides is estimated to affect a quarter of the world’s human population.34 Ascaris is a geohelminth, meaning that part of its lifecycle occurs in soil. Ova require warm, moist soil to embryonate into infective larvae. In ideal settings, Ascaris ova can persist for over 14 years. Therefore, this parasite flourishes in tropical areas where year-round transmission is possible. However, its distribution is global and it has been found in up to 4% of refugees from Eastern Europe.19 Transmission is facilitated by crowded living conditions and breakdowns in sanitation. Worm burdens are highest in 5–15-year-olds.34 Geophagia, the intentional consumption of soil, is a common behavior of children in endemic areas and has been shown to greatly increase both the risk of harboring any Ascaris worms and the risk of having large worm burdens.35
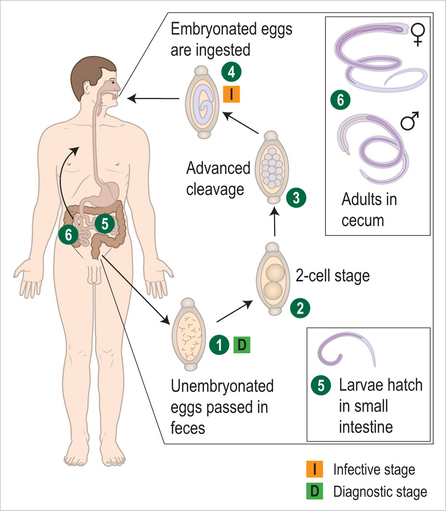
Life cycle:
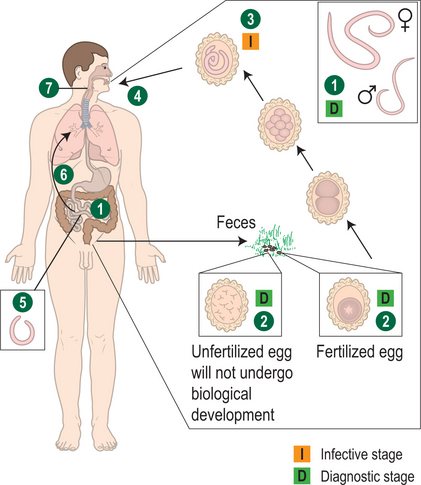
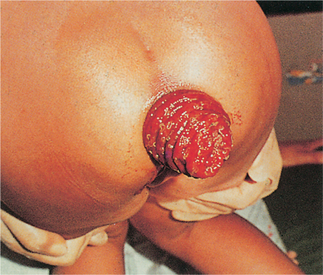
Humans are infected through ingestion of embryonated eggs in contaminated soil. The larvae hatch in the small intestines, penetrate the intestinal wall, and migrate to the lungs via blood or lymphatic vessels within 4 days after ingestion. While developing in the alveoli for approximately 10 days, the larvae can cause a transient respiratory syndrome, characterized by fever, cough, wheezing, and radiological pulmonary infiltrates, known as Loffler’s syndrome. Loffler’s syndrome can be caused by other intestinal nematodes with a similar transpulmonary migration, such as hookworms and Strongyloides. The pulmonary symptoms, if present, rapidly resolve as the larvae leave the lungs through the bronchi and then are swallowed. After returning to the intestines the larvae develop into adult worms. Within 2–3 months, gravid females start releasing 200 000 fertilized ova per day. Adult worms live for up to 2 years and then are passed in stool. In individuals infected with only male worms, no ova will be detected (Fig. 20.3). If the migrating larvae gain access to the systemic circulation, they may cause local symptoms resembling those of visceral larva migrans caused by Toxocara canis, the dog roundworm, which is discussed below.36
Clinical manifestations:
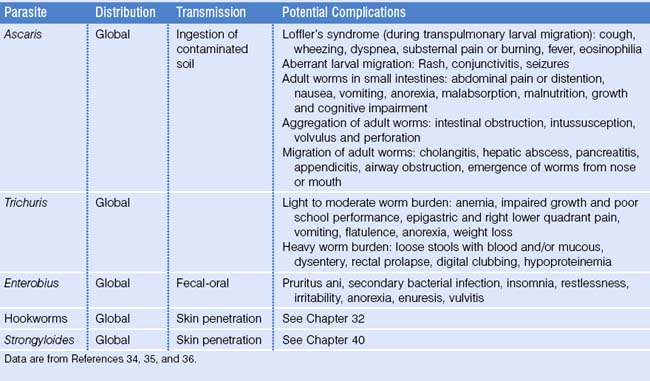
The vast majority of infections go unnoticed. However, there is considerable evidence that even these apparently asymptomatic infections can lead to poor growth in children via decreased appetite and malabsorption.34 A variety of other nonspecific symptoms may be caused by worms within the intestines (Table 20.3). Both children and adults may suffer from serious acute complications. Over half of these complications occur when a mass of worms within the small intestines leads to intestinal obstruction.34 This typically occurs in 1–5-year-old children with large worm burdens and may present with colicky abdominal pain and emesis. Occasionally, adult worms are seen in the vomitus. In some areas of the world, intestinal obstruction from Ascaris worms is the leading cause of acute abdominal emergencies (Fig. 20.4).37 Other acute complications, including cholangitis, hepatic abscess, pancreatitis, and appendicitis, occur when adult worms migrate into various locations. Complications may arise many years after immigration if remnants of deceased worms or eggs remain in the biliary system and elicit the formation of calculi. For example, recurrent pyogenic cholangiohepatitis has been reported in a Vietnamese-American woman approximately 15 years after emigration.38
Diagnosis:
Diagnosis is made by finding characteristic eggs in the stool, or passage of worms in vomitus or feces or via the nose or mouth. Diagnosis of the acute complications may be facilitated by imaging studies. Eosinophilia is commonly seen only during larval migration through the lungs.
Etiology:
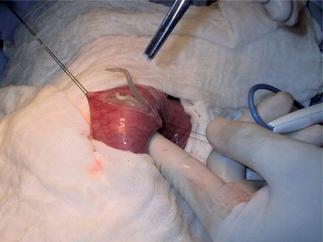
Trichuris trichiura, commonly called whipworm due to its unique morphology, is a ubiquitous geohelminth (Box 20.2; Fig. 20.6). These worms live in the cecum, but may cover the entire colon in heavy infections. The presence of visible worms on the mucosa of a prolapsed rectum is pathognomonic for trichuriasis.
Box 20.2 Case study 2
A 6-year-old girl presents to an emergency room for evaluation of diarrhea. She was born in a refugee camp in Thailand and moved to the United States 4 months ago. Her parents bring her to the emergency room after having several small bowel movements of mucus and blood. She is afebrile and has a moderate amount of pain in her right-lower quadrant. The physician notices a prolapsed rectum (Fig. 20.5).
Epidemiology:
Like A. lumbricoides, T. trichiura thrives in climates with abundant rainfall, high humidity, and shade. However, it occurs in individuals throughout the world, including 8% of refugees from Eastern Europe.19 Over one billion people worldwide are estimated to be infected with this organism.35 The highest worm burden is seen in school-aged children. Geophagia increases the risk of Trichuris infection.35
Lifecycle:
Its method of transmission is similar to A. lumbricoides, the ingestion of mature eggs via fingers contaminated with infected soil. However, after the larvae hatch they remain in the intestine as they develop into adult worms, lacking a transpulmonary migration (Fig. 20.7). Given the similar mode of transmission, it is not surprising that children are often coinfected with Ascaris.
Clinical manifestations:
Most T. trichiura infections involve relatively few worms and the vast majority of these light infections are thought to be asymptomatic. However, there is considerable evidence that, even with light to moderate worm burdens, children may suffer from anemia, impaired growth, and poor school performance.35 Several non-specific signs and symptoms may include abdominal distension and pain, especially in the epigastric and right iliac fossa areas, vomiting, flatulence, anorexia, and weight loss. These gastrointestinal symptoms may be more likely in individuals coinfected with Ascaris and/or hookworm.
Heavy infections can cause significant morbidity. Dysentery with mucus and blood in stools is a common presentation that is often accompanied by rectal prolapse. Dysentery can be made worse by coinfection with protozoa such as Entamoeba histolytica and Balantidium coli or by dysentery-associated bacterial pathogens. In severe infections, young children may develop digital clubbing and hypoalbuminemia.36 Intermittent painless hematochezia has been reported in adult immigrants.39 The differential diagnosis of the Trichuris dysentery syndrome includes amoebic colitis. Trichuriasis may also present similarly to acute appendicitis with right-lower quadrant abdominal pain (see Table 20.3).
Diagnosis:
Trichuriasis is not associated with prominent eosinophilia since there is no tissue migration of the worms. Diagnosis can be made in heavy infections by visible worms on a prolapsed rectum or by proctoscopy. Colonoscopy has also diagnosed symptomatic infections.39 Typically, diagnosis is made by detecting the eggs, with characteristic bipolar plugs, on stool O&P examination.
Other nematodes
Hookworm and Strongyloides are covered in detail in separate chapters.
Enterobius vermicularis:
E. vermicularis, commonly known as pinworm, is found throughout the world. It occurs more often in children and among individuals living in crowded living conditions, such as orphanages.40 The prevalence among immigrant groups is unknown since screening stool O&P examinations have an extremely low sensitivity for detecting this organism (<5%).36 It is primarily transmitted via fecal–oral contamination. Adult female worms live in the cecum and migrate to the anus at night to deposit eggs on the perineum. Adult worms only live for 1–3 months, but persistent infections are common due to repetitive infections resulting from ongoing fecal–oral spread among affected individuals, facilitated by the pruritic response to the deposited eggs. Other means of transmission include contact with soiled bed linens and inhalation of contaminated dust.36
The most common symptom is pruritus ani caused by the irritating effects of the deposited ova (see Table 20.3). Secondary bacterial infections may develop if excoriations are severe. Several nonspecific symptoms include insomnia, restlessness, irritability, anorexia, and enuresis. In females, E. vermicularis can occasionally cause vulvitis with associated mucoid discharge and itching. Diagnosis relies on detection of eggs on cellophane tape placed in the perianal area or by visualization of adult worms near the anus at night.36
Larval nematode infections
Toxocara canis:
The dog roundworm has a global distribution, and has a similar lifecycle to Ascaris. Dogs ingest soil containing eggs, and puppies can become infected in utero by transplacental transmission. When humans, typically young children, accidentally ingest eggs, the larvae do not develop into adult worms. Instead, they wander throughout the body causing local inflammation in various tissues, especially the liver and eye, before becoming phagocytosed.36 Most human infections are asymptomatic. However, children with heavy infections may present with findings of the visceral larva migrans (VLM) syndrome: fever, wheezing, hepatomegaly, and occasionally symptoms of heart failure, seizures and/or focal neurological deficits. Laboratory findings include marked eosinophilia, leukocytosis, and increased serum globulins. Ultrasonography may reveal hypoechoic liver lesions. Most VLM cases spontaneously resolve after 2 years, but some children may die without treatment. Another potential presentation is ocular toxocariasis. Affected children often present with strabismus or a white pupil, leukocoria, due to a retinal granuloma. Imaging studies should be performed to differentiate these granulomas from retinoblastomas. Occasionally, extensive intraocular inflammation can occur leading to retinal detachment. Detection of Toxocara antigens in the serum and/or vitreous fluid by enzyme-linked immunosorbent assay (ELISA) facilitates the diagnosis.36
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree