Hepatocellular carcinoma (HCC) is the sixth most common cancer for both sexes or the fifth most common cancer for men worldwide with 749,000 new cases diagnosed in 2008 (5.9% of all cancers).1 Furthermore, it is the third leading cause of cancer death, responsible for 695,000 deaths in 2008.1 Although HCC is still relatively uncommon in the United States, the incidence of HCC is projected to increase, given increasing rates of chronic hepatitis C virus (HCV) infection.2 Despite the rising incidence of primary liver cancer, the vast majority of liver cancers are metastases (1.8 million cases/year) with colorectal cancer being the most common.3,4
Curative treatment for both primary liver cancer and isolated liver metastases is surgical resection. However, very few patients are considerd medically operable. As a result, several interventional oncology technologies have emerged over the past few decades for the treatment of liver cancers. Herein, we review the basic principles of transcatheter and ablative therapies currently in practice.
Hepatic arterial embolization for the treatment of hepatomas was first reported in 1977 by Yamada et al,5 with safety and efficacy demonstrated in the 1980s and more refined transarterial regimens created over the last 20 years. Today, several transarterial therapies exist with the primary goal of local control as a bridge to resection/transplantation or as a palliative treatment. These therapies exploit the dual blood supply of the liver with tumors receiving the majority of their blood supply from the hepatic artery whereas normal nontumorous liver receives the majority from the portal vein.6 Thus, by selectively occluding hepatic arterial vessels that preferentially supply the tumor, transarterial therapy begins a cascade of events leading to cell death via ischemic necrosis in tumor nodules, while selectively sparing normal tissue supplied by the portal vein. There are many flavors of transarterial therapy, which span from bland embolization with microparticles to chemoembolization with a mixture of chemotherapeutic agents, to radioembolization where resin or glass microspheres impregnated with radioactivity are delivered to the tumor.
Prior to any method of transarterial therapy, anatomic characterization with either CT or MRI is critical for preprocedure planning. Both CT and MRI are used to predict normal and/or anatomy and extrahepatic feeding vessels to help plan a general approach, which is ultimately mapped out using angiography at the beginning of each case.7,8
Until recently, portal vein thrombosis was considered an absolute contraindication to transarterial embolic therapies. However, in 2005, a small, 32 patient series demonstrated that these patients may be successfully and safely treated, without hepatic infarction or failure.9 Nonetheless, treatment in these patients should proceed with care, especially since outcome prediction remains challenging, despite the authors suggesting either the Child–Pugh class9 or the Milan criteria10 be used for this purpose.
In addition to preprocedure imaging, analysis of liver function tests is critical. In order to avoid postprocedure acute liver failure, careful analysis of liver function pre- and projected post-procedure is performed to ensure adequate functioning residual tissue both via laboratory values and volume-based analysis. Such analysis is multifactorial, dependent not only on tumor burden, but also on patient size, function of background liver, portal vein involvement, and other medical comorbidities.7
In the postprocedure setting, there are a number of complications that can occur including liver failure (0% to 49%), hepatic abscesses (<10%), biliary strictures (<10%), ischemic cholecystitis (<10%), and gastritis with ulceration/bleeding (3% for bleeding).7 However, postembolization syndrome, characterized by fever, fatigue, and occasionally abdominal pain, is most common, occurring in 60% to 80% of patients.7 It is usually self-limiting and resolves in anywhere from 3 days to 1 week. An associated transaminitis is also usually transient, with only 5.7% of 351 patients experiencing permanent liver function deterioration in a series following 942 transarterial chemoembolization (TACE) procedures.11 Nonetheless, despite limited morbidity, it may lead to longer hospital stays for pain control.
Transarterial embolization (TAE) or bland embolization refers to embolization using particulates, which are not mixed with chemotherapeutics or other drugs. Bland embolization has been used for both unresectable HCC (Fig. 126-1) and recurrent HCC after partial hepatectomy with multiple small trials demonstrating safety and efficacy (Table 126-1). This therapy became a well-accepted therapy in 2002, when Llovet et al12 conducted a large prospective randomized trial in which TAE (bland embolization) versus TACE versus best supportive care was compared in a head to head trial for patients with unresectable HCC. The trial demonstrated a clear survival benefit of TAE and TACE over supportive care and as a result was terminated prematurely for ethical reasons.
FIGURE 126-1
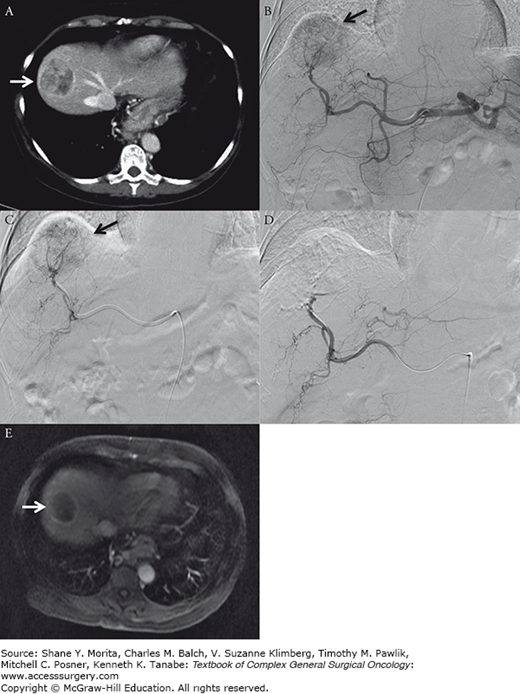
Transarterial embolization. Sixty-one-year-old female with severe COPD and HCV cirrhosis presents with 4.8 cm segment 8 hepatocellular carcinoma. Patient was treated with transarterial embolization in an attempt to maintain her on the transplant waiting list. A. Preembolization arterial phase contrast-enhanced CT demonstrates enhancing segment 8 mass (white arrow). B. Proper hepatic arteriogram demonstrates prominent segment 8 tumor blush (black arrow). C. Selective embolization of right hepatic artery branch supplying tumor (black arrow). D. Postembolization right hepatic arteriogram demonstrates successful occlusion of tumor feeding vessels with notable absence of tumor blush. E. Postembolization T1-enhanced MRI performed 1 month following treatment demonstrates decreased size and lack of internal enhancement within tumor (white arrow).
Review of Seminal Data
| Modality | Study | Design | Results |
|---|---|---|---|
| Conventional TACE | Llovet et al12 | Intention to treat survival analysis RCT; 112 patients with unresectable HCC, Child–Pugh A/B and Okuda I/II; TAE, doxorubicin TACE or supportive care | Terminated early for ethical reasons due to clear survival benefit; survival % at 1 and 2 years for TAE, TACE, and supportive care: 75/50, 82/63, and 63/27 |
| Conventional TACE | Lo et al21 | Survival analysis RCT; 80 Asian patients with unresectable HCC; cisplatin/lipiodol TACE or supportive care | Survival % at 1, 2, and 3 years for TACE and supportive care: 57/31/26 and 32/11/3 |
| Conventional TACE | Georgiades et al9 | Prospective cohort; 32 patients with unresectable HCC and portal vein thrombosis treated with TACE | Median survival 9.5 months; no TACE-related hepatic failure or infarction; survival % at 6, 9, 12, and 18 months: 60/47/25/12.5 |
| DEB-TACE | Lammer et al (PRECISION V)41 | RCT assessing tumor response (EASL) at 6 months; 212 patients Child–Pugh A/B, unresectable HCC, N0, M0; conventional TACE or DEB-TACE using doxorubicin | Complete response, tumor response, and disease control in DEB-TACE vs. cTACE: 27/52/63 and 22/44/52, without significance; significant decrease in serious liver toxicity and doxorubicin-related side effects with DEB-TACE |
| Radioembolization | Salem et al45 | Prospective cohort; 291 HCC patients treated with Y90 | Response rate % (WHO/EASL): 42/57; time to progression: 7.9 months; survival time (months) for Child–Pugh A and B: 17.2 and 7.7 |
| Radioembolization | Lewandowski et al47 | RCT assessing ability to downstage disease; 86 patients with T3 HCC; TACE or Y90 treatment | Y90/TACE for partial response %: 61/37; downstaging %: 58/31; event-free survival (months): 17.7/7.1; overall survival: 41.6/19.2 |
| Radioembolization | Gray et al51 | Phase III RCT; 74 patients with bilobar unresectable liver metastases from colon adenocarcinoma; SIR–Spheres plus hepatic arterial chemotherapy or hepatic arterial chemotherapy alone | Y90 plus HAC/HAC for partial and complete response by tumor volume: 50/24; CEA 72/47; time to disease progression by tumor volume: 12.0/7.6; 1-, 2-, 3-, and 5-year survivals: 72/68, 39/29, 17/6.5, 3.5/0; no increase in grade 3 to 4 toxicity or loss of quality of life |
| Radiofrequency ablation | Mazzaferro et al69 | Prospective cohort; 60 HCCs in 50 cirrhotic patients awaiting OLT underwent single session RFA | Complete response %: 55 and 63 for tumors ≤ 3 cm; tumor persistence % at 12 and 18 months: 59, 70; post-OLT 3-year survival %: 83 |
| Radiofrequency ablation | de Baere et al75 | Prospective cohort; 121 treated hepatic metastases in 68 patients | Eradication rate: 91% in 100 metastases followed for 4 to 23 months; tumor control equivalent for percutaneous and intraoperative RFA; local recurrence rate: 9% in median 79 days |
Transarterial embolization may be performed with a variety of different embolic agents including gelatin sponge, acrylic copolymer gelatin, polyvinyl alcohol (PVA), and newer spherical gelatin microspheres, which come in various sizes (i.e., 40 to 1200 μm). The specific choice of embolic agent is dependent on the tumor size and vascularity with the ultimate goal of a distal tumor bed embolization to prevent collateral vessel formation. Importantly, the use of smaller size particles is more effective in terms of causing ischemic necrosis and tumor cell death due to more distal penetration into the tumor’s vascular bed. However, there is a fine balance between adversely affecting the tumor and harming neighboring liver and/or nontarget organs. For example, gel powder was found to cause biliary strictures and bile duct cysts despite reaching smaller vessels and thus is no longer used.13–15 Another consideration is permanent versus temporary arterial occlusion. Agents such as PVA, microspheres, and steel coils are permanent, whereas the gelatin sponge is temporary.16,17 While gelfoam and PVA particles are traditionally considered permanent agents, one of the advantages over coil embolization is the ability to recannulate previously embolized vessels in the setting of recurrence, something which can be next to impossible after coil embolization. Given these considerations, the most popular agents are gelatin sponge and PVA particles.18
Conventional TACE (cTACE), the combination of embolic materials and chemotherapy by suspension, provides optimal localization of the chemotherapy by occluding the feeding vessel and allowing higher dose administration and longer dwell times in the tumor tissue than can be achieved systemically. Given these benefits, some centers predict that TACE should theoretically provide a higher survival benefit than TAE and argue that hypoxic change in the absence of chemotherapy may inhibit apoptosis, stimulate angiogenesis, and thereby promote tumor growth.19,20 However, since Llovet et al12 terminated their trial prematurely, there is no level I evidence demonstrating survival benefit with the addition of chemotherapy to TAE and the decision to utilize chemoembolization is often center and operator dependent.
In addition to Llovet et al,12 another prospective randomized trial by Lo et al21 demonstrated that cisplatin TACE provides clear survival benefit to patients with unresectable HCC when compared to supportive care. The response rate to TACE as judged by tumor necrosis and burden has been shown to range from 15% to 61%.22–28 Given the overwhelming level I survival benefit data, a consensus statement in 2010 named TACE as part of the standard of care for intermediate/advanced HCC treatment.29
In addition to unresectable HCC, for cases of resectable HCC with greater than a 5-cm mass or multiple smaller masses in the absence of vascular invasion or distant metastases, TACE has been demonstrated to improve survival outcomes when performed before primary resection or orthotopic liver transplant (OLT). In 1996, a study based in Milan demonstrated that patients with a solitary tumor less than 5 cm or three or fewer tumors less than 3 cm without gross vascular invasion had favorable survival outcomes post-OLT, a finding that was validated after a review of nearly 3000 transplants.10,30 Patients treated within the Milan criteria had twice the 5-year survival rate as those who fell outside of the criteria. Downstaging patients to within Milan criteria for transplant became important when studies revealed that patient survival post-downstaging was determined by the downstaged value rather than the initial stage.31–33 Thus, TACE has helped many patients qualify for transplant. Such promising results have made TACE one of the most popular transarterial therapies for HCC. TACE is also often used as a bridge to transplant in patients on the transplant list based on a theoretical potential benefit, when patients are left waiting for an average of 64 days for a liver.30 However, currently no clear survival benefit or reduced dropout rates have been demonstrated when it is used as a bridge to transplant in these patients who do not require downstaging in order to qualify for transplant.34
Several chemotherapeutic agents have been combined with embolic agents in TACE. Such agents include doxorubicin, epirubicin, aclarubicin, 5-fluorouracil, mitomycin, cisplatin, and styrene maleic acid neocarzinostatin. There is currently no available data to demonstrate any single or combination of therapies to be superior.35 Worldwide, the most popular therapy is doxorubicin monotherapy. In the United States, the most popular therapy is combination therapy with doxorubicin 50 mg, cisplatin 100 mg, and mitomycin C 10 mg.
In addition to chemotherapeutics, many techniques involve the addition of lipiodol (Guerbet Laboratories, Roissy France), an iodinated ethyl ester from poppy seeds that was found to improve chemotherapy localization by improving tumor retention. The lack of Kupffer cells in tumor nodules delays the clearance of lipiodol, which is a delivery vehicle for chemotherapeutics; as a result, the addition of lipiodol is thought to increase the exposure of tumor tissue to the intended drug. Lipiodol has also been shown to increase antitumor properties for advanced HCCs that have some portal venous supply.36,37 Lastly, because lipiodol is radio-opaque, it stains the tumor and makes targeting in future treatments technically easier, thus providing both therapeutic and practical benefits. If added to TACE, lipiodol is mixed with the chemotherapeutic agents by pumping methods, which creates a uniform emulsion for injection. The optimal ratio of lipiodol to doxorubicin is 2–3:1 in order to create a water-in-oil emulsion.38
In this technique, drug-eluting beads or microspheres are chemically loaded with chemotherapeutic agents to provide a more controlled release of drugs to the tumor with decreased peak plasma levels. Drug-eluting bead-transarterial chemoembolization (DEB-TACE) is a relatively new technique under investigation, with phase I and II data demonstrating safety and efficacy.39,40 Early data from a prospective randomized trial (PRECISION V) comparing cTACE to DEB-TACE demonstrated a decreased incidence of severe liver toxicity, lower doxorubicin-related side effects, and a higher mean total dose of doxorubicin.41 In addition, results trended toward higher objective response with statistically significant improved response in bilobar or recurrent disease (Child–Pugh B, Eastern Cooperative Oncology Group (ECOG) performance 1). Additionally, a retrospective case control study demonstrated improved survival.42
The most common type of DEB-TACE currently in practice is doxorubicin loaded onto PVA microspheres by immersion. Although other microspheres are also available that can load doxorubicin or cisplatin. Doxorubicin-loaded beads are most commonly used for HCC as well as a number of other metastatic liver diseases including neuroendocrine tumors. Recently, irinotecan-loaded beads have been demonstrated to have a tolerable safety profile for palliative treatment of metastatic colorectal cancer.43
Radioembolization refers to the administration of microspheres loaded with Yttrium-90 (Y90). Y90 is a radioactive element that emits beta-radiation as it decays with a half-life of 64.2 hours. The two most widely used Y90 beads (usually 20 or 40 μm) are TheraSpheres (glass microsphere, MDS Nordion, Kanata, ON, Canada) and SIR-Spheres (resin microsphere; Sirtex Medical, Lane Cove, Australia).
Radioembolization has been U.S. Food and Drug Administration (FDA) approved for both HCC and metastatic colorectal cancer.44 The largest series to date (291 patients with unresectable HCC) demonstrated an overall tumor response rate of 42% using World Health Organization (WHO) criteria (complete and partial response), with particular benefit in those with Child–Pugh A disease.45 Radioembolization has been demonstrated to help slow the progression of hepatocellular carcinoma and thus keep patients on the transplant list while they wait for an available organ.46 Radioembolization has also been shown to increase transplant eligibility by downstaging patients by Milan criteria47 with a trend toward superiority over TACE. Pathologic correlation has demonstrated high rates of tumor necrosis, with 89% complete histologic necrosis in tumors less than 3 cm.48 However, unlike with TACE, downstaging via radioembolization has no proven survival benefit, although radioembolization has been shown to provide a survival benefit in a subset of patients with vascular invasion and portal vein thrombosis (which remains a relative contraindication for TACE).49 The possibility of radiation segmentectomy, or the radioembolization of two or less hepatic segments, using much higher doses of radiation than typically used for radioembolization, has also been explored. Median doses as high as 1214 Gy have been delivered to tumor tissue with only 210 Gy delivered to the infused normal hepatic parenchyma (accounting for tumor hypervascularity), with a relatively high safety profile.50
For patients with metastatic disease, studies have demonstrated similar favorable data. In 2001, Gray et al51 demonstrated favorable survival data for 74 patients with metastatic colorectal cancer in a phase III randomized trial when SIR-Spheres were combined with intrahepatic floxuridine (FUDR) as compared to FUDR alone, with increased tumor response rates and times to progression. Since then, several studies have demonstrated efficacy for metastatic colorectal patients.52 Fewer studies have reported results with other metastases; however, in 2005, Popperl et al53 and Lim et al54 published separate results demonstrating favorable outcomes in a series of 23 and 46 patients, respectively, including several different types of liver cancer. Also in 2005, Coldwell and Nutting55 reported that all of 34 patients with unresectable metastatic breast cancer demonstrated decreased size and number of tumors via PET/CT analysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






