INTRODUCTION
In head and neck cancer patients, complex care issues exist that are best addressed by an interdisciplinary approach. Disabilities that can result during and after treatment affect eating, swallowing, speech, and daily social contact, all of which can severely affect a patient’s overall quality of life (QOL). Physical, social, and psychological well-being are greatly affected by the potential disfigurement and dysfunction resulting from disease and its treatment.
1Several modalities of treatment of head and neck cancer are well established. Radiation therapy, surgery, chemotherapy, and various combinations of these three therapeutic modalities all comprise today’s optimal treatment.
2,3 Investigative treatment methods include biological therapy, immunotherapy, and gene therapy. The treatment regimen for a specific patient depends on tumor site, stage, histology, and other factors. Treatment is composed of a single-modality or a combined approach. Each treatment method entails both general symptom management and specific care related to its distinctive side effects.
This chapter presents guidelines for interdisciplinary symptom management for patients receiving treatment for cancers of the head and neck. We begin with a discussion of what constitutes interdisciplinary care, drawing in part on the experience of collaboration at an urban Cancer Center, a center of excellence in head and neck cancer. A discussion of fatigue, a common and troubling symptom, is followed by a section on nutrition. Pain assessment and management, including algorithms to assist in treatment, skin care, and the management of mucositis follow. We have included discussion of psychiatric conditions, psychosocial assessment, the incorporation of integrative medicine in oncology as well as issues of survivorship. Finally, we have included a section on the integration of palliative care within oncology practice, with evidence to suggest this as a best practice model.
INTERDISCIPLINARY SYMPTOM MANAGEMENT
Comprehensive symptom management that anticipates patient needs helps patients and their families participate in care, complete all treatments without interruption as recommended by their health care team, and adjust to changes in function and appearance brought about by cancer and its treatment.
3 Providing comprehensive symptom management to head and neck cancer patients is uniquely challenging, but is critical to successful treatment outcomes.
The identification of potential problems, as well as individual strengths, should be made at the time of initial contact with patients. These may include physical, psychological, spiritual, practical, social, and cultural issues. A review of information regarding cancer diagnosis, plan of care, treatment-related side effects, and their management may be presented by several providers, before treatment actually begins. Identifying factors which may affect patient learning (e.g., educational level, language barrier, and unfamiliarity with the medical system) can be identified and addressed proactively.
Multimodal treatment for head and neck cancer requires coordination from each member of the interdisciplinary team, especially when care is provided across several ambulatory sites (e.g., radiation oncology and chemotherapy sites) as well as inpatient settings. At the Continuum Cancer Centers of New York, a video conference connecting three physical sites is held weekly.
4 The goal of this conference is to maximize the communication and coordination between providers, by reviewing each head and neck cancer patient’s progress through treatment. Disciplines represented include advance practice nurses from radiation oncology, ambulatory and inpatient medical oncology nurses, an integrative oncology nurse, social workers, nutritionists, speech and swallowing specialists, and palliative care specialists. The presence of significant physical symptoms, psychosocial distress, and unusual family circumstances which may impact care are discussed.
In addition to this conference, an interdisciplinary team from three sites, consisting of psychiatrists, social workers, nutritionists, palliative care specialist, and patient advocate collaborates weekly. Individual patients are discussed, as well as sharing of resources and expertise. The time spent on this interdisciplinary communication and collaboration improves patient care, reassures patients that providers are communicating with each other, and assures safe transitions in level of care, especially between inpatient and outpatient settings.
Fatigue
Fatigue is a common state in patients with head and neck cancer. Although its mechanism of development is undoubtedly multifactorial, it is thought that a variety of circumstances, such as growth of the cancer itself, chemotherapy, radiation therapy,
compromised nutrition, physical “deconditioning,” altered sleep patterns, hormonal changes, anemia, worry, and fear all contribute to a feeling of overwhelming tiredness or exhaustion that interferes with QOL and daily functioning.
5 Although being “tired” is part of the human condition, its intensity is often greatly amplified when the fatigue is induced by cancer or its treatment.
The underlying mechanism causing fatigue is receiving greater attention as survivors of head and neck cancer live longer and experience more intensive multimodal treatments. It has been proposed that fatigue is the result of the production of proteins by actively growing cancer cells. These cytokines or cell proteins may be responsible for fatigue as well as weight loss; both important nonspecific constitutional symptoms that guide clinicians’ judgment about a patient’s response to treatment or underlying disease progression.
6Attempts to successfully address fatigue can help sustain the QOL experienced by patients and their families. A variety of pharmacologic and nonpharmacologic interventions may alleviate patients’ fatigue or restore a patient’s sense of control over their symptoms and environment. Several factors may contribute to fatigue in the head and neck cancer patient. These include physical decondition, sleep, hormonal changes, and anemia, which are elaborated below, along with nutritional factors as well as mood and anxiety which are addressed in subsequent sections.
Physical Deconditioning
Energy conservation, a balance between physical (and mental) activity and rest may be both protective and restorative in the midst of, or after multimodal treatment for, head and neck cancer. Patients who overextend themselves describe compensatory periods of rest in the following days. Counterintuitively, an optimal amount of physical activity helps maintain energy rather than deplete it. Self-directed home-based programs or those tailored to patient needs include flexibility training or yoga.
7 Referral to a physical medicine and rehabilitation specialist can help customize a plan for recovery. Special emphasis on the flexibility of the neck and jaw can lead to fewer long-term consequences.
Sleep Regulation
Too much sleep, too little sleep, or poor-quality sleep can account for a component of the fatigue experienced by head and neck cancer patients.
8 Pain and difficulty clearing oral secretions commonly interrupt sleep in head and neck cancer patients. Patients may even avoid sleep for fear of aspirating. Elevation of the head of the bed, or sleeping in a recliner may alleviate this component. Restorative sleep is an important component of the healing process.
Hormonal Changes
Hypothyroidism is a well-known sequela of radiation therapy to the head and neck area and needs to be monitored in long-term survivors. Patients whose treatment is limited to surgery to the thyroid or parathyroid similarly need periodic monitoring and adjustment of thyroid replacement. The systemic influence of other hormonal changes, particularly testosterone in men and estrogen in women, may play a significant role in overall energy reserves that are depleted by cancer and its treatment.
Anemia
The contribution of anemia to fatigue and its potential correction with erythropoietin-
α has elevated anemia from a rarely considered state to its current prominence. Guidelines for the correction of anemia to physiologic levels are changing with greater use and outcomes evaluation in head and neck cancer survivors. Iron supplementation is often unnecessary and ineffective to correct fatigue. Correction of anemia may help improve QOL by allowing patients to remain active and functional.
9 Until recently, supplementation of endogenous erythropoietin had been thought to optimize outcomes, especially in QOL, but Henke found that patients with advanced head and neck cancer (T3, T4, or nodal involvement) who received 300 U/kg of erythropoietin-
α with 60 to 70 Gy had achieved loco-regional control correlated negatively with the expression of erythropoietin receptors.
10
Depression
Low energy is a symptom of depression. A full psychiatric assessment of a patient with fatigue is an important facet of adequately addressing the symptom which may derive from or contribute to an underlying mood disturbance which is commonly experienced in head and neck cancer patients (please refer to Psychiatric Considerations section).
NUTRITION
The head and neck cancer patient is particularly challenged within the greater cancer population when it comes to sustaining adequate nutrition. This is due in part to the physical location of the cancer itself and its proximity to the mouth and entire upper digestive tract. The long list of potential nutritional problems starts with the head and neck and works its way through the digestive tract for this patient population, who should be closely monitored for symptoms arising throughout treatment. Following a diagnosis, surgical interventions, radiation, and chemotherapy courses can and do have immediate and lasting impact on one’s ability to take food and liquids orally.
11 Ongoing consultation with an oncology dietitian/nutritionist is essential in guiding patients throughout treatment and beyond.
Screening and Assessment of Nutritional Status
Patients may or may not present with preexisting weight loss and/or malnutrition. Some patients enter into treatment reportedly asymptomatic. Others present with diminished ability to eat “normally” due to pain, dysphagia, or oral intake issues due to tumor location or previous surgery. Similarly, some patients will already have a percutaneous endoscopic gastrostomy (PEG) tube in place to facilitate adequate nutrition where a compromised oral route exists.
Patients initiating a treatment regimen of chemotherapy, radiation therapy, or a combination of both require screening and a comprehensive nutritional assessment as early as possible, ideally prior to the start of treatment. Clinicians can assist in screening a patients general nutritional status during an initial consult by noting the following and referring for nutrition intervention and follow-up as indicated:
Screening
Age and visual exam
Diagnosis, preexisting disease (i.e., diabetes), s/p head and neck surgery
Weight/height—Underweight/obese/body mass index (BMI)
Weight history—Usual body weight/weight change
Oral symptoms: Ability to eat, dentition, dysphagia, mucositis, odynophagia, etc.
GI symptoms: Nausea, vomiting, diarrhea, constipation, PEG
Appetite and intakes (food recall for adequacy of calories/protein/fluids/nutrients)
Social support: Ability to shop/purchase/cook and store foods
Lab values
It is common for patients to express a significant loss of appetite and even aversion to foods, thereby making it challenging to maintain adequate nutrition. Nutrition intervention and counseling can help the patient maintain an optimal weight, facilitate tolerance and healing from treatment, minimize symptoms, maintain or improve QOL, and decrease morbidity.
Nutritional Needs
During a baseline (or initial) nutrition assessment, the dietitian/nutritionist estimates the patients nutritional needs and provides guidance and specific written information to the patient and their family and/or caregivers. At the minimum, the precachectic patient requires adequate calories and protein to facilitate weight maintenance and healing. Encouraging the patient to continue eating their preferred foods is stressed; however, adjusting food intakes to meet an individual’s increased nutritional needs must also be a consideration. Undergoing chemotherapy and radiation treatment increases the bodies need for calories (kcal), protein, and fluids.
12 General guidelines are as follows:
Patient remain on a regular, nutrient rich, weight-maintaining diet
Caloric needs: 30 to 40 kcal/kg body weight
Initiate meal replacement drinks BID in addition to regular meals (see Supplementation section)
Protein needs: 1.3 to 2.0 g/kg body weight
Fluid needs: Usually 1 cc per kcal; however, will be dependent on chemotherapy regimen, hydration status, and lab values.
Modified food texture/composition based on symptom management and specific patient needs (i.e., soft, moist food, nonacidic, liquid diet)
Enable patient access to foods (i.e., financial assistance, enteral food ordering)
It is worth briefly noting the myriad of symptoms that comprise cancer cachexia in this section about estimating nutritional needs. Cachexia involves metabolic abnormalities and inflammatory processes beyond what is seen in starvation, for example, the loss of lean body mass and production of cytokines. The addition of calories alone will not reverse the loss of weight and muscle mass in the cachectic patient. Clinicians are challenged in recognizing those patients at risk based on their symptoms. Researchers are seeking a consensus that defines cachexia
13 to facilitate best practice for its treatment,
14 and a variety of pharmacological treatments are being explored for efficacy.
Nutritional Supplements
For patients undergoing chemotherapy and radiation to the head and neck, symptoms can appear gradually and worsen as treatment progresses. Clearly, this will affect the type and texture of food and liquids that can be consumed orally. It is critical to emphasize the additional intake of high-calorie, high-protein food and fluids prior to becoming symptomatic (see recommendations above). Early intervention with nutritional supplementation can reduce the chance of inferior outcome in patients at high risk for weight loss.
15Most cancer centers have a multitude of commercial meal replacement drinks at their disposal to provide to patients as samples, such as Ensure, Boost, Orgain, and the like. Most standard supplements have between 250 and 375 calories and between 9 and 15 g of protein per 8-oz can or box. Patients should be encouraged to test different brands and flavors for tolerance and palatability. If a supplement is too dense, it can be thinned with liquid or blended into a milkshake. Patients may augment their diet with supplements between meals or as meal replacement when solid foods are too difficult to consume. Ready-todrink supplements are convenient to use if a patient has a long travel time to treatment as they do not require refrigeration prior to opening. They are also useful for patients lacking the energy to shop and/or prepare foods at home. Most grocery stores carry generic meal replacement drinks as well as a variety of prepared juice blends or protein-based drinks, which can contain as many calories and protein as commercial supplements.
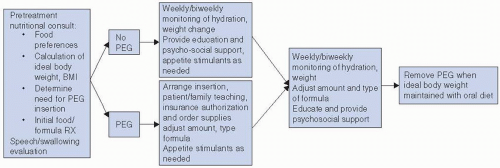
PEG Tube
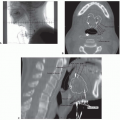
Some head and neck patients may require a PEG tube during the course of their care (
Fig. 10-1). This may be due to an extensive surgery or a compromised or unsafe oral route. The purpose of the PEG is to facilitate provision of adequate liquid food (enteral food) and fluid to the patients GI tract when oral ability is compromised, contraindicated, or significantly diminished. It is a clinical decision to determine whether a patient may require a PEG emergently during the treatment course or if it should be placed prophylactically based on the anticipation of significant
toxicities. More research is necessary to inform physician behavior on whether prophylactic PEG tube placement is warranted in the treatment of head and neck cancer.
16 In any event, the PEG is a valuable tool for maintaining nutrition in patients whose symptoms prevent adequate oral intakes, thus risking significant weight loss. Such a group of patients requires specialized care from their health care team in regard to nutritional support. It is standard to initiate a bolus feeding with a 1.0 to 1.5 kcal/cc enteral formula that contains fiber. Formula selection is tailored to medical history (i.e., diabetes), and patients should be closely monitored for tolerance and appropriate usage. Method of feeding and formula can be modified as needed based on tolerance. PEG feeding frequency should increase as oral intakes diminish; however, oral intake of tolerated foods is encouraged to continue as long as the swallowing ability is safe.
Counseling and Education
Nutrition education is an extremely helpful means for patients and their family to actively participate in their own care. Ongoing counseling throughout treatment enables clinicians to address issues as they arise. In oncology centers without an oncology dietitian on staff, patients should be screened and receive general nutrition advice from RNs and/or other clinicians. Simple handouts with age and education-level appropriate ideas for high-calorie/high-protein, soft, nonacidic food and liquids can be provided. Patients should be made aware of the normal symptoms they may experience and provided with the tools to minimize them, such as the appropriate diet modifications and/or medications. A combined approach of medications for symptom management and nutritional counseling is utilized.
If patient face-time is limited, develop a resource handout that lists recipes, cookbooks, and websites that are specific to optimizing nutrition through head and neck cancer treatment.
PAIN ASSESSMENT AND MANAGEMENT
Pain is a significant problem that affects 50% to 80% of patients with cancer and disturbs overall QOL.
17 Yet cancer pain can be effectively managed in up to 90% of patients by following certain guidelines of pain management.
18 Pain in the head and neck patient can be disease-related or treatment-related or a combination of both.
17,19,20 Nearly all patients who receive radiation therapy to the head and neck experience pain at some point during treatment.
19,20 It is important to institute analgesics early in treatment in order to maximize patient comfort.
The assessment of pain should not only include the descriptive dimensions of the pain (location, intensity, quality, temporal features of onset and daily fluctuations, and aggravating and alleviating factors), but assessment of cause and effect on functioning. Once a cause has been identified, disease-modifying treatments may be offered, for example, the use of radiation for bony metastases. A description of burning or shooting pain, for example, can guide treatment toward use of adjuvant medications for neuropathic pain. A thorough initial pain assessment is important to ascertain past use of analgesics and effectiveness as the treatment of opioid-tolerant patients is significantly different than opioid-naive patients.
21Awareness of psychosocial aspects of the experience of pain is an important component of assessment: What is the meaning of pain for this patient and family? What cultural, religious, or spiritual dimensions are influencing perception of pain and attitudes toward treatment?
18 For minority patients with pain, significant barriers to effective pain management may exist, including beliefs about stoicism and difficulties communicating with physicians and other health care providers.
22 The ongoing assessment and management of pain includes the psychosocial impact of pain on the patient and family, quality and nature of distress, and the assessment of psychiatric comorbidities, for example, anxiety and depression, as well as history of substance abuse.
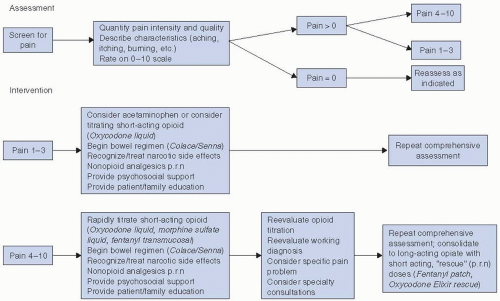
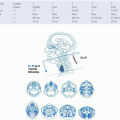
Pain management for patients with head and neck cancer should follow established guidelines, based on the original principles elucidated by the World Health Organization (WHO) and the Agency for Health Care Policy and Research (AHCPR).
19,23 These are briefly reviewed in
Figure 10-2. Small doses of opioids, for example, morphine or oxycodone, available in either pill or liquid form, may often safely and effectively be used for the treatment of moderate pain.
Evaluation of pain should be part of every assessment as a standard of care.
24 The goal of pain management is to provide sustained comfort with few side effects and in this way to maximize patients’ QOL. The treatment of cancer pain commonly includes the use of opioid analgesics, but other categories of treatment may also be included; for example, nonopioid analgesics and other adjuvant medications as well as interventional therapies, neurostimulation, and integrative medicine approaches.
24 The use of nonsteroidal anti-inflammatory drugs (NSAIDs, e.g., naproxen or celecoxib) must be done cautiously due to safety concerns of gastrointestinal and cardiovascular risks.
Frequent reassessment of the analgesic regimen is important to evaluate efficacy and tolerability. Titration of current medications, rotation to other opioids, and/or addition of adjuvant medications may be required, especially if pain has nociceptive and neuropathic components.
18 For more severe and prolonged pain and for patients with history of substance-use disorders, methadone may be used. It is a long-acting opiate that can treat both the incident and neuropathic components of cancer pain. Methadone has a long half-life averaging about 24 hours, can be dosed in 6-to-8-hour intervals, and compared with other opiates is relatively inexpensive. Due to lack of active metabolites, it may be preferable in the setting of renal impairment.
24 In view of its prolonged half-life, as well as individual variation, familiarity with prescribing is essential, as full effects of the medication may be delayed for days and up to a week or more. Patient and family education is essential to reinforce the idea that methadone is not only for maintenance but also for withdrawal from heroin.
Nonopioid Analgesics
Drugs used to treat the neuropathic component of pain are often part of the analgesic regimen, as nerve involvement is common in cancer pain and treatments. The use of gabapentinoids (gabapentin or pregabalin), glucocorticoids, such as prednisone or dexamethasone, as well as bisphosphonates for bone pain has been well studied.
24Patients may have inadequate pain relief due to lack of knowledge, fear of narcotic dependency, fear of acknowledging disease progression, or poor clinical management.
25 Lack of adherence to medication regimens has been studied, with evidence suggesting that around-the-clock (ATC) dosing is more frequently adhered to than as-needed (PRN) and that patients are not always prescribed ATC medications, according to practice guidelines.
26 They may also have comorbid conditions that exacerbate cancer pain. These issues need to be considered in order to achieve maximum patient comfort.
Postoperative pain is initially controlled with parenteral narcotics, administered by continuous intravenous route, patient-controlled analgesia (PCA), or by subcutaneous injection, converted to an oral analgesic via the nasogastric tube or by mouth. For patients who have been treated with opioid analgesics prior to surgery, informing the acute pain management team prior to surgery is important, as these patients may need more than usual amounts of postoperative analgesics. Mild pain should be treated by acetaminophen-based
products, rather than aspirin or nonsteroidal anti-inflammatory medications, which can reduce platelet aggregation and impede healing or optimal cosmesis.
Moderate levels of pain can be initially managed with codeine-based analgesics,
27 or combinations of acetaminophen and opioids in small doses. Severe pain should be treated with regimens that include opioids (morphine, oxycodone, oxymorphone, or fentanyl-based regimen). With any class of opiates, analgesics with a short half-life should be prescribed based on their period of effectiveness, usually every 3 hours to establish needs, then converted to longer acting preparations orally or parenterally. Longer acting opioids are usually given every 8 to 12 hours, depending on patient response.
Because taking oral medications can be difficult during treatment of head and neck cancers, the transdermal fentanyl patch may be a good choice; however, this is not advised as initial therapy. Patients should begin with short-acting preparations to assess appropriate requirement and tolerance. Typical doses for the transdermal fentanyl patch range from 12 to 100 mcg per hour, but may be titrated up to 200 to 300 mcg per hour. A new patch is applied to clean, dry skin every 3 days; patients are given instructions on how to apply new patches, cautioned about dangers regarding extreme heat (e.g., sun exposure, fever), which can create irregular dose absorption, and educated about slow onset of action.
Drugs for Breakthrough Pain
Often as regimens are being titrated, or due to the episodic nature of the pain, short-acting drugs are required in between the longer acting opioids. This can be either single-agent formulations, such as morphine, oxycodone, hydromorphone, or combinations with opioids and nonopioid. Fentanyl formulations, which are absorbed quickly, are now available in the form of lozenges, buccal tablets and patches, sublingual tablets, and nasal spray.
24 Regular breakthrough doses signal a need for higher doses of the long-acting preparation, unless the cause is expected to resolve quickly. The dose of the short-acting drug is generally roughly 5% to 15% of the total daily dose, though recommendations are to begin with the lowest dose of fentanyl preparations, as the effects of these medications may not be proportional to the fixed-dose scheduling.
18,24Assessment and treatment of severe and refractory pain may require continuous narcotic intravenous infusion delivered through a subcutaneous peripheral site or centrally through an indwelling central venous catheter. A patient-controlled analgesia (PCA) pump is commonly used, most often in hospital settings, because it delivers maintenance (“basal” rate) of analgesia plus a patient-controlled “rescue” dose for breakthrough pain. Morphine, hydromorphone, fentanyl, and methadone are common drugs used for PCA infusion. This method is used frequently for patients with end-stage disease.
Neural blockade approaches and implant therapies encompass a varied group of medications. Neurosurgical pain control for refractory pain and neuropathic pain in specific areas, for example, the brachial plexus, or for cancer-related facial pain may be effective, using chemical or radiofrequency neurolysis.
28With any opiate, care must be given to monitor constipation and sedation, as well as mouth dryness or urinary retention, which may require change in dose, preparation, or delivery route. When initiating any narcotic analgesic, patients are instructed to increase fluid intake by at least 1 L per day and to use a docusate-based stool softener liberally and daily to prevent constipation, often with addition of a laxative. Patients who can take medications by mouth should be instructed to take the medication with food or a supplement to minimize potential nausea.
Education of patients and family regarding pain management is important.
27 As health care has changed and patient care has shifted more to the outpatient area and to patients’ homes, knowledge of how to manage pain must be provided to caregivers. Mode of action, expected side effects of pain medications, and implementation of a bowel regimen are important aspects of patient and caregiver teaching. Written and verbal instructions and information should be given.
Risk Assessment and Addiction Issues
A routine barrier to adequate analgesic treatment is the fear that opiates of any class when used for cancer pain from illness or treatment will become addictive. Patients with a preexisting dependence or substance abuse themselves are the most vulnerable patients, and their management should respect both this predisposition and their need for adequate analgesia.
29 Ironically, patients who have been dependent on opiates, barbiturates, or benzodiazepines in the past likely will need higher than expected doses for pain relief. Such a contradictory pattern mandates good communication and very clear dosage parameters. This involves a minority of patients who are overrepresented among those with head and neck cancers who have a higher incidence of substance abuse. For the majority of patients without substance abuse histories, fears of addiction may interfere with using adequate amounts of analgesics. Reassuring patients that tolerance to a drug is not a sign of drug addiction is very important. Educating medical professionals about the important distinctions between drug tolerance, dependence, and addiction is also important as misconceptions about these terms and their meaning may be barriers to optimum prescribing of the most effective analgesic regimen.
For patients at risk for ongoing substance abuse, referrals to and collaboration with addiction specialists may be a necessary and important part of treatment. The use of additional methadone for pain management in patients who are already enrolled in methadone maintenance treatment programs for heroin addiction may be safer than using other opioids. Other risk assessment strategies may be employed, for example, dispensing smaller quantities with frequent monitoring and screening of urine for prescribed and nonprescribed drugs.
Residual Pain
Neuropathic pain throughout the face, especially throughout the ear and the mandible, serves as a constant reminder of their cancer to a minority of patients who have undergone multimodal treatment. Often mechanical in origin with resulting nerve compression or irritation, these symptoms are treated with customdesigned oral appliances, exercises, or anticonvulsants. Patients benefit from ongoing assessment with speech and swallow specialists for exercises to maximize function in the face of more chronic, residual pain.
Tricyclic antidepressants and anticonvulsants have been shown to be effective in neuropathic pain syndromes and are a reasonable alternative depending on comorbidities.
30 Duloxetine, a serotonin reuptake inhibitor antidepressant, has been approved for use in diabetic peripheral neuropathy and may be helpful in cancer or chemotherapy-related neuropathic syndromes as well.
30,31 Regular evaluations by a dentist or oral surgeon is often indicated to rule out persistent or recurrent cancer.
SKIN CARE
Managing skin reactions of patients undergoing therapy is crucial in minimizing patient discomfort and pain. Preventing breaks in treatment which may affect treatment outcomes for patients with head and neck cancer is another reason skin care management is so important. Though there is a paucity of evidence-based literature regarding care of the skin, some principles of skin assessment and management are clearly appropriate.
A radiation skin reaction is an expected, normal, and temporary side effect of radiation therapy. It is also the most frequently experienced side effect of radiation therapy, affecting between 87% and 90% of all patients receiving radiation.
32 The reaction is caused by the effect of radiation on the normal process of epidermal cell turnover. The mitotic ability of stem cells within the basal layer of epidermis is damaged by ionizing radiation. This decreases repopulation of cells which are then more rapidly shed.
33 This causes a thinning of cornified cells at the skin surface, making the skin more vulnerable to further radiation damage. An inflammatory response leads to release of serotonin and histamine and resulting edema. Increased blood flow to the injured tissues leads to erythema. Melanin rises to the surface of the skin, causing the characteristic darkening of the radiated skin.
33,34 Alopecia is common as the hair follicles are affected and is permanent after 5 to 6 weeks of treatment.
34Standards of care for managing radiation-induced skin reactions are based primarily on clinical judgment and expert clinician preferences. Randomized clinical trials are few and far between and have not demonstrated strong evidence for any particular skin care regimen.
33,34 Development of standards for skin care, based on what evidence is available, is strongly encouraged.
Assessing patients’ skin before radiation begins is an important first step in skin care management. This involves evaluating patients’ individual risk of skin breakdown during treatment, due to both patient-related and treatment-related factors (
Table 10.1). The entrance and exit portals should be evaluated for obvious
breaks in skin integrity. Skin folds, lips, and ear lobes are at increased risk of breakdown, as is the thin, sensitive skin of the eyelids.
The acute reaction begins 2 to 3 weeks after starting radiation and continues 3 to 4 weeks after completion of therapy.
32,33 The effect of the radiation on the skin is cumulative and progresses from mild erythema through hyperpigmentation to dry desquamation (dryness, pruritis) and moist desquamation. Moist desquamation, in which loss of the epidermis leads to open, weeping skin, can occur in skin fold areas (ear lobes) and at the point where two treatment fields overlap. Moist desquamation places patients at risk for infection and fluid losses and can cause significant anxiety and discomfort.
33,34 Severe moist desquamation can lead to a treatment break, which may affect the overall effectiveness of radiation treatment.
35Providing clear, comprehensive patient education on skin care measure to minimize pain, decrease incidence of skin breakdown, and decrease infection is vital.
32,34 As most skin care measures are done by patients, it is important to obtain their active involvement. Detailed verbal and written instructions for skin care during treatment should be provided to patients. This should begin with a review of what patients may expect, a rough timeline of when each phase of the reaction will most likely appear, and the interventions for each phase. Changes in appearance in each phase as well as information on possible discomfort and pruritis should be described. Reassurance that any discomfort will be addressed promptly is also important.
32As previously stated, the evidence for skin care management is sparse and conflicting, without clearly proven “best practices.” However, those skin care measures which
are generally agreed upon are described in
Table 10.2. Cleansing the skin within treatment fields involves washing the skin gently with warm water, using only mild soaps (e.g., Dove unscented or Basis soaps), and rinsing well before gently patting the skin dry.
36 A gentle shampoo (e.g., a baby shampoo) is also recommended.
37Moisturizing skin within the treatment field using a hydrophilic moisturizer (e.g., Aquaphorâ, Eucerinâ, aloe vera gels) is also generally recommended.
32,33,34,37 Patients should apply moisturizer two to three times per day (depending on risk of breakdown of specific tissue), being careful to wash any residual moisturizer off the skin before treatment. On any day on which treatment is not given (weekends, holidays), moisturizer should be applied after washing in the morning and again at bedtime. Patients should be instructed to prevent irritation of radiated skin by avoiding physical irritants (e.g., tight fitting clothing and shaving), chemical irritants (e.g., perfumes, cosmetics,
deodorants, and moisturizers other than those recommended), extreme heat or cold, and prolonged, direct sun exposure.
32,33,34Though study patients were women receiving breast irradiation, a recent prospective, double-blinded, placebo-controlled study was done to compare effectiveness of three different skin care products versus placebo in reducing the incidence of radiation-induced skin reactions.
38 Products evaluated included Aquaphor ointment, Biafine RE cream, RadiaCare gel, and a sterile water mist—the placebo. Despite a more-than-adequate sample size and well-executed blinding of researchers, the study revealed no product to be more effective than a sterile water mist in preventing skin reactions.
38 The study did support the evidence that patients prefer using some type of product (ointment, cream, or gel) to water.
Several RCTs of the efficacy of aloe vera gel
39,40,41 have revealed no statistically significant advantage of aloe vera over routine skin care. Biafine has not been shown to provide a statistically significant advantage over cheaper, more available products,
42 and in a more recent study comparing Biafine with calendula (marigold extract) crème, Biafine was found to be less effective.
43Calendula and hyaluronic acid may be somewhat effective in reducing the incidence of radiodermatitis,
44 but more studies are needed to support this. Interestingly, a study done by Wells
45 demonstrated no statistical differences in skin reaction of patients using aqueous creams, sucralfate creams, hydrogels, versus no cream/gel at all. Statistically, increased skin reactions were seen in smokers, patients getting chemotherapy, and patients getting bolus treatments (additional therapy to a specific area).
During radiation therapy, patients’ skin is assessed for the degree of skin discomfort, presence of scaling or pruritis, tissue breakdown, or moist desquamation. Patients receiving concomitant chemotherapy, cranial radiation, or nasopharynx radiation may note peeling of the scalp, pruritus, and alopecia. Such patients are instructed to use a mild shampoo and to frequently apply the recommended moisturizer to the scalp.
46 Any signs and symptoms of skin infection (fever, increased pain, purulent, or foul-smelling discharge) should be evaluated promptly, drainage should be cultured if present, and antibiotics should be prescribed.
32,33,46 Skin care continues after radiation therapy has completed. This includes assessment of skin reaction resolution, reinforcement of the need for lifelong prevention of further skin injury by use of sun block (sun protection factor [SPF]-45), and the avoidance of extreme heat or cold to skin in the treated area.
Long-term effects of radiation therapy on skin are noted months or years after completion of radiation therapy and may include thinning of epithelium (with increased risk of skin injury), telangectasia, loss of sweat and sebaceous glandular function, and hyperpigmentation or hypopigmentation. These effects are difficult to predict and are often more related to individual patient characteristics than to radiation dose or technique.
47