Abstract
Spinal cord injury due to cancer is common and can lead to deficits in sensation, strength, and function. Patients that develop spinal cord injury due to cancer have unique rehabilitative needs that vary based on the severity and location of spinal cord injury. These patients are also at risk of unique medical complications as a result of their spinal cord injuries. This chapter will discuss the functional and medical considerations of these patients and their need for inpatient rehabilitation.
Keywords
Cancer, Durable medical equipment, Family meeting, Function, Inpatient rehabilitation, Pain, Spinal cord injury, Spinal tumors
Introduction
A spinal cord injury (SCI) is a life-changing event. Regardless of the cause or the time course of presentation, the patient will be affected dramatically as will his or her support system.
Perhaps the most important aspect of rehabilitative care of a person with SCI due to cancer is to understand the goals of inpatient rehabilitation (IPR) in this population. These goals can be affected by diagnosis, with, for example, the needs of a person with complete C4 tetraplegia are much different than that of a person with incomplete T11 paraplegia, but also by prognosis. A patient with an excellent prognosis could maximize his/her time in IPR in order to try and achieve a goal of going home with independence or modified independence. However, for a patient with a prognosis of less than 6 months, IPR of SCI would be better focused on family training, patient education, and discharge home as soon as possible with focus of optimizing the remaining quality of life. These goals and lengths of stay vary from situation to situation, but it is generally accepted that with appropriate family involvement and training for care of a person with SCI due to cancer, length of stay can be as short as 10 days or less.
All patients discharged from IPR require a plan for ongoing care called a discharge plan. The goal of which is to anticipate not only the current medical and physical needs of the patient but also those medical and physical needs that will exist or develop in the coming months and design a plan to meet those needs. The rehabilitation team determines, in collaboration with the oncology team, who can give insight into the specific prognosis for cancer progression, a projection of functional recovery or decline which in turn dictates durable medical equipment (DME) and ongoing therapy needs post discharge. Successful implementation of the discharge plan is affected by a host of factors. For example, patient resources can play a role in discharge planning and continuity of rehabilitative care after discharge. In some cases, a patient may require a hospital bed, commode, or transfer assistance device such as a hydraulic patient lift for safe discharge home, but this DME may not fit inside the home or may be unaffordable, making a safe discharge plan less than optimal. Another example is that a lack of access to an automobile or public transportation can affect a plan for continued rehabilitation in an outpatient setting after discharge.
The successful rehabilitation of a person with SCI due to cancer relies upon teamwork and constant communication between the rehabilitation team, the patient, and the family. Ultimately, IPR is only the first step in an arduous road to medical, physical, and psychologic recovery or decline and a manageable death.
Spinal Cord Injury and Cancer Diagnosis
Tumors of the spine often lead to SCI; tumors of the spinal cord by definition cause SCI. Any part of the spinal cord can be affected and to varying degrees. As such patients can present with complete or incomplete paraplegia or tetraplegia. SCI related to tumors and cancer of the spine is most often due to direct compression, although vascular insults are possible as well.
Persons with SCI due to cancer often present with a variety of symptoms including those common to any cancer patient such as pain, fatigue, malaise, and weight loss in addition to neurologic dysfunction including focal weakness of one or more extremities, difficulty walking or standing, sensory losses, dysesthesias, and bowel and bladder incontinence or retention. These neurologic signs and symptoms may present insidiously or appear suddenly. Pain related to SCI and cancer can be localized to the spine at the level of the tumor or metastasis or may appear in a dermatomal or radicular distribution. Back pain related to cancer is often characterized as dull or achy, which is not an uncommon presentation for noncancer-related back pain; however, if a patient complains of back pain that is worse at night or with lying down, clinicians should have a high suspicion for cancer as a cause.
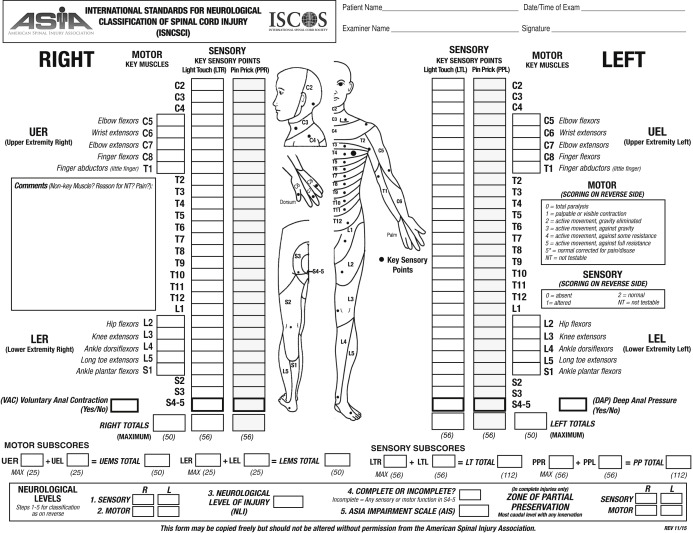
A patient’s history is also important in diagnosing SCI due to cancer. For example, a patient with a prior history of cancer merits workup of possible metastases to the spine when presenting with the aforementioned symptoms. First and foremost a thorough physical examination is important in diagnosing spinal metastases or tumors. A full neurologic examination should be completed and should include strength examination and sensory examinaton and should include a digital rectal examination to test for sensation, tone, and volitional contraction. The International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) is an examination used to quantitatively score the motor and sensory impairment, determine a neurologic level of injury, and determine the severity of SCI by assigning an American Spinal Injury Association Impairment Scale (AIS) ranging from “A” to “E” ( Fig. 10.1 ). Persons without any sensation or motor function in the lowest sacral dermatomes (S4-5) would be classified under the 2011 ISNCSCI revision as having a complete injury and designated AIS “A.” Those who have sensory function in the lowest sacral dermatomes (S4-5) but no motor function more than three levels below the neurologic level of injury would be classified as having an incomplete sensory injury and designated as AIS “B.” Persons with sensory function in the lowest sacral dermatomes (S4-5) and motor function more than three myotomal levels below the motor level would be classified as having an incomplete motor injury and designated as AIS “C,” or “D.” If at least half of the key muscles, where a single key muscle is defined for each of five upper extremity myotomes from C5-T1 and five lower extremity myotomes from L2-S1 on both sides of the body, below the neurologic level of injury have a muscle grade strength of at least 3 out of 5, the AIS would be designated “D,” if not the SCI would be designated as AIS “C.” The AIS “E” designation is reserved for those with SCI who make full recovery of motor strength and sensation within all dermatomes and key muscle myotomes. The ISNCSCI examination is utilized to track neurologic function and should be performed at minimum yearly on patients with SCI to ensure no neurologic deterioration requiring neurologic workup. Special consideration and more frequent ISNCSCI examinations should be performed on those patients with SCI due to cancer as these patients are prone to progressive or worsening SCI by the very nature of their disease state. Should neurologic deterioration be discovered in a patient with SCI due to cancer, immediate workup including imaging should be performed.
After obtaining a thorough history and physical, if a clinician has suspicion that his or her patient has cancer or a tumor that is causing or has the potential to lead to compromise of the spinal cord, the next diagnostic step should be urgent imaging. Imaging should include magnetic resonance imaging (MRI) and computed tomography (CT) of the area of the spine (cervical, thoracic, or lumbosacral) thought to be affected. MRI is the best modality for evaluation for neural compromise, whereas CT is the best modality for evaluating bony destruction and spinal stability. MRIs should be ordered with contrast since almost all intrinsic spinal cord tumors and metastases enhance with gadolinium. Should the MRI elucidate tumor or metastatic lesion, MRIs of the remaining segments of the spine should be considered to rule out other tumor or metastasis along the spine as well. Positron emission tomography (PET) scans are mostly used for surveillance and are not used to diagnose cancer of the spine but can be used to assess for any new metastatic disease.
Spinal Cord Injury and Cancer Prognosis
With improved medical and oncologic care, persons with cancer are living longer with less cancer-related morbidity. However, this also means that some persons are living with deficits and issues that previously would have been untreatable. This is apparent in the reported 18,000 new cases of spinal tumor per year in North America of which 85% are metastatic. Cancer is thought to metastasize to the spine either via the lymphatic or vascular system. Some studies suggest that up to 70% of those with cancer will have metastases to the spine. In fact, spinal tumors account for 26% of nontraumatic SCI admissions to IPR units. Cancer of the spine can be primary (cancer or tumor arising in the spine) or secondary (metastatic). The most common forms of cancer that metastasize to the spine are lung, breast, kidney, prostate, and thyroid. Spine lesions are most commonly diagnosed in the thoracic spine, despite cadaveric studies showing they are more common in the lumbar spine. This may be due to the narrowed spinal canal and vascular anatomy making mass lesions in this area more likely to lead to symptoms of complete or incomplete paraplegia.
Spinal tumors can be classified into three man subtypes by location: intramedullary, intradural-extramedullary, and extradural. Different subtypes of tumor have different prognoses and different types of tumor or cancer (See Chapter 8 for more information on tumors of the spine). It is important to note that given that intramedullary tumors arise from within the spinal cord itself, even a small intramedullary tumor can have dramatic neurologic effects.
Spinal tumors, by nature of their classifications, cause SCI and neurologic dysfunction in different ways. The most common cause of dysfunction due to primary tumors (intramedullary or intradural-extramedullary) involves direct destruction of the nervous system by tumor invasion. The most common cause of dysfunction due to secondary tumors (metastatic disease) is compression of neural tissue within the spinal canal. Metastatic lesions of the cervical and thoracic spine are most often the result of lung and breast disease, whereas lumbosacral involvement is most often due to prostate, colon, or pelvic involvement. Survival rates for common metastatic cancers to the spine include the following:
- •
Lung metastases to the spine with 50% survival rate at 1 month and at best 16% after 24 months.
- •
Breast cancer with metastases to the spine with 44% survival rate after 24 months.
- •
Prostate cancer with metastases to the spine with 25% survival rate at 24 months.
- •
Patients with primary spine tumors who completed IPR programs had a median survival of 9.5 months with 1-year survival of 47.4% and 5-year survival of 10.5%.
- •
Patients with secondary spine tumors had a median survival of 2.8 months with 1-year survival of 21.4% and 5-year survival of 3.6%.
Cancer may also cause SCI indirectly. According to the American Cancer Society, the incidence of cancer from 2008 to 2012 was 454.8 per 100,000 persons. With cancer being so prevalent, these indirect causes of SCI become more common as well.
Paraneoplastic syndromes are a group of syndromes that are an autoimmune response to a primary cancer or tumor. Via this pathway these syndromes lead to medical issues at sites of the body distant from the cancer’s actual location. Paraneoplastic syndromes have been known to cause cerebellar degeneration, myasthenia gravis, as well as transverse myelitis (TM), which is an inflammatory disorder of the spinal cord that results in SCI. In over 50% of cases of TM, a specific cause of the inflammatory condition is not found; however, paraneoplastic syndrome should be considered within the differential diagnosis of a patient presenting with TM of unknown etiology, especially if this patient has a history of cancer.
Treatment of cancer can also cause SCI. Radiation-induced SCI is a possibility when radiation is used in the treatment of cancer adjacent to the spinal cord. Irradiation of lung cancers and cancers of the head and neck leave the spinal cord particularly susceptible to radiation-induced SCI. Depending on the anatomic area irradiated, different levels of the spine (cervical, thoracic, or lumbosacral) could be affected. As such, radiation-induced SCI could lead to paraplegia or tetraplegia. Radiation-induced SCI can present in multiple fashions. It could present as a transient myelitis, a limited SCI that most often presents 2–4 months after irradiation and most commonly presents with electric shock–like neuropathic pain and can also be accompanied by varying degrees of weakness. Transient myelitis induced by radiation is self-limiting and does not require treatment to resolve. Despite the self-correcting nature of transient myelitis, recovery can take up to 40 weeks, and depending on level of impairment, these patients could benefit from intense rehabilitation. Patients who have had irradiation to treat cancer can also develop chronic progressive radiation myelopathy (CPRM) that most often first appears 9–15 months following irradiation, but has been reported to present as long as 3 years following irradiation. CPRM is hallmarked by demyelination of the spinal cord leading to white matter necrosis and atrophy of the spinal cord itself. Radiation can also lead to vascular injury in the spine leading to SCI. The likelihood of developing CPRM is thought to be related to the volume of the spinal cord exposed to radiation and the total dose of radiation received. The accepted dosing of radiation to the spinal cord is not to exceed 45 Gy, although if it is necessary for successful treatment of the cancer, a small volume of cord is allowed to receive a higher dose. Studies have shown that patients that received 50–55 Gy to the spinal cord had a 1% chance of developing CPRM after 2 years, whereas those that received 55–60 Gy to the spinal cord had a 5% chance of developing CPRM after 2 years, and those that received 68–73 Gy to the spinal cord had a 50% chance of developing CPRM. It is generally accepted that the risks of radiation to the spinal cord outweigh the benefits above 60 Gy. There is no treatment known to reverse CPRM, and neurologic dysfunction is usually permanent.
Secondary Conditions of Spinal Cord Injury and Cancer
In order to provide the most effective IPR of a patient with SCI due to cancer, it is important to be aware of the secondary conditions unique to SCI and how to best manage these. These medical considerations can vary based on the type of cancer diagnosis and location; however, there are some more commonly experienced medical conditions that will be discussed in the following section.
Neurogenic Bladder
Before discussing neurogenic bladder, and neurogenic bowel, further, it is important to understand that patients with SCI can present with either upper motor neuron (UMN) or lower motor neuron (LMN) injuries. It is important to differentiate the two types of SCI in order to provide the proper medical management. Lower motoneurons are cells whose axons leave the central nervous system (CNS) and connect to skeletal muscle fibers. LMN dysfunction arises from damage to the anterior horn cells within the spinal cord, specifically to the cell bodies of the LMNs or to the motoneuron axons within the cauda equina. With injury to LMNs, affected muscles are typically flaccid and there is loss of reflex activity. UMN dysfunction arises from damage to neurons proximal to the LMNs which reside entirely within the CNS. Reflex activity typically is preserved below the level of injury for UMN injuries. Most SCIs with the exception of injuries to the conus medullaris, such as may occur with a chordoma, or cauda equina compression, or cord infarction in which segments of the cord are damaged due to ischemia can be expected to present as UMN injuries. In order to assess for a UMN SCI, the practitioner should complete an anal examination that includes assessing for bulbocavernosus and anocutaneous reflexes. If these reflexes are intact and there is normal resting or increased anal sphincter tone on physical examination, then a patient has a UMN SCI. If these reflexes cannot be elicited and the anal sphincter is flaccid on examination, then a patient has an LMN SCI. With few exceptions, those persons with tetraplegia or mid- to high-level paraplegia have UMN injuries and those with low-level paraplegia have UMN, LMN, or a combination of injuries.
Neurogenic bladder is common after SCI, and lack of proper management can lead to significant morbidity with life-threatening infections and even renal failure. A neurogenic bladder can be either a UMN or LMN type, and the management approach for each type is different; however, if there is any doubt about the appropriate bladder management for a patient with SCI, it is always acceptable to insert a Foley catheter transurethrally and consult urology for further recommendations. Consultation to and follow-up with a urologist with experience in neurogenic bladder is standard of care for any SCI patient.
Upper motor neuron bladder management
Those patients with UMN lesions and neurogenic bladder typically have a spastic detrusor muscle (the muscle within the bladder wall responsible for contracting and expelling urine) and spastic urinary sphincter (the muscle responsible for maintaining continence at rest and allowing expulsion of urine by relaxing during normal voiding). This invariably results in variable degrees of detrusor sphincter dyssynergia, a condition in which a spastic detrusor muscle contracts but the sphincter does not relax at the same time. When a spastic detrusor muscle is triggered to contract against a closed sphincter the pressures generated within the bladder may exceed the capacity of the sphincter to maintain closure allowing urine to be expelled and incontinence especially if bladder sensation is also impaired. If the pressures are not excessive (<50 cm H 2 O) and most of the urine is expelled leaving a post void bladder residual volume less than 200–250 mL, then this involuntary reflex emptying of the bladder can be considered “safe” or “balanced.” If the pressures are sustained and exceed 50 cm H 2 O, the risk for upper urinary tract deterioration with the potential for the development of hydronephrosis and renal insufficiency increases and reflex emptying alone would not be considered a “safe” bladder management technique. In this latter case or in the situation where urine is largely or completely retained within the bladder, emptying of the bladder with a urinary catheter is essential.
There are four basic ways for patients with UMN neurogenic bladder to empty: normal voiding, reflex voiding, intermittent catheterization, and continuous indwelling catheter drainage with a Foley catheter. Reflex voiding is a reasonable goal for a male patient without normal control whose bladder empties involuntarily or with incomplete control at safe pressures as noted earlier. In this situation, the individual would continuously wear a condom catheter over his penis. In order to ensure this management remains “safe,” periodic bladder residual urine checks (several time daily in the beginning) should be performed, the serum creatinine monitored (weekly in the beginning), bladder pressures assessed with urodynamic study (if there is any suspicion that voiding is not “safe”), upper urinary tracts imaged (at baseline and yearly) with a renal sonogram to screen for hydronephrosis, and the patient queried for the presence of autonomic dysregulation (discussed in autonomic dysregulation section) as well as the frequency of urinary tract infections. Should a patient managing his bladder with reflex voiding have low 24-h output in the drainage bag attached to the catheter (indicating perhaps the patient is retaining urine) or elevated bladder volumes on scanning for residual urine within the bladder, then the patient should be intermittently catheterized. Many patients with SCI will develop worsening spasticity over the first few months after SCI that will make it more likely for them to be able to reflex void. Also α-1 blockers, such as tolterodine, can be used to “relax” the urinary sphincter presumably decreasing sphincter outlet resistance and improve the chances of successful reflex voiding.
Intermittent catheterization is the preferred method of management for persons, both men and women, who retain urine and are able to perform the technique safely themselves. It is typically performed every 4–6 hours depending on the volume consumed and the bladder capacity before reflex voiding is triggered. It is important to note that new onset leaking in between catheterizations can be caused by urinary tract infection, overingestion of liquids, and evolution (changing spasticity) of bladder after SCI. If a patient is leaking in between catheterizations and the bladder capacity before reflex voiding is triggered is low (<400 mL), a bladder-specific anticholinergic, such as oxybutynin, or a β 3 adrenergic agonist, such as mirabegron, can be added and titrated to promote continence.
Indwelling catheters are indicated for those individuals who retain urine and are unable to perform intermittent catheterization or for those who reflex void without control and are unable to maintain an external urine collection appliance and are at risk for skin breakdown.
Lower motor neuron bladder management
LMN bladders are flaccid by nature and treated almost exclusively by intermittent catheterization every 4–6 hours.
Special considerations
A patient with a lesion causing progressive mass effect or a patient that could develop new metastatic lesions has the potential to have changing bladder management needs over time, and as such should be followed closely by a urologist and physiatrist. A patient with SCI due to cancer prognosis is also a consideration. If a patient has a poor prognosis (on the order of a few months), an indwelling catheter is often chosen for quality-of-life reasons.
Neurogenic Bowel
Neurogenic bowel is also common after SCI and like neurogenic bladder, it also has different treatment considerations based on whether a patient has UMN or LMN SCI. Having control over when a patient might have a bowel movement not only allows therapy sessions to go uninterrupted but also allows the patient to learn to have more control over his or her life and avoid embarrassing situations. A patient with UMN bowel can be considered to have regular, albeit slow, functioning of his or her intestines through the transverse colon. This is due to the fact that the gastroenterologic (GI) tract from esophagus to splenic flexure is innervated by the vagus nerve, whereas the pelvic and pudendal nerves innervate the GI tract for the remainder of the colon and rectum. Connections to these lower nerves are often interrupted in SCI. The goal for these patients is to have soft, but formed stool. These patients require stool softeners, laxatives (osmotic or irritant), and suppositories or mini-enemas in combination with digital stimulation in order to evacuate their bowels completely. An example of a medication regimen for these patients would be
- •
Docusate sodium 100 mg by mouth twice daily
- •
Senna 17.2 mg by mouth at bedtime
- •
Bisacodyl suppository with digital stimulation daily
When scheduling these medications, it is important to note that one should expect the laxative (in the above example senna) to take approximately 8 h to work. As such, the suppository should be scheduled accordingly. The combination of the suppository and digital stimulation of the anus takes advantage of the recto-colic reflex that is still intact in a person with UMN SCI. Other reflexes can also be leveraged, such as the gastrocolic reflex with bowel routines being scheduled after breakfast or dinner. Patients with sufficient manual dexterity and with the use of specialized commodes can learn to perform their own suppository insertion and digital stimulation. Many patients with UMN bowel will not have the manual dexterity to perform their own bowel routines. In these scenarios, a family member or caregiver should be trained to perform the bowel routine. Patients with LMN injuries have a flaccid bowel. As such they do not respond to irritant laxatives, suppositories, or digital stimulation. In addition, these patients have a flaccid sphincter and are prone to accidents or anal leakage. With this in mind, the goal for these patients is to have hard stool formed in the shape of small pebbles. These patients normally do not take medications, but could take stool softeners or stool-bulking agents, such as psyllium, as needed to obtain the appropriate stool texture. Persons with LMN SCI require manual disimpactions for bowel management. These patients are prone to accidents after meals, and for that reason they may need to perform several bowel routines per day. Most patients with LMN bowel are able to perform their own bowel routines; however, should they not be able to for any reason, a caregiver or family member should be trained on how to do so for that patient. There are special considerations for those patients with SCI secondary to cancer. A patient with a lesion causing progressive mass effect or a patient that could develop new metastatic lesions has the potential to form changing bowel management needs over time and as such should be followed closely by a physiatrist. Patients on chemotherapy may develop nausea or diarrhea. They may also be on opioid medications for pain control that can slow bowel transit time for these patients even further. Laxatives, stool softeners, and stool-bulking agents should be adjusted as needed.
Spasticity
Patients with UMN SCI typically will develop spasticity. Spasticity is a form of hyperreflexia manifesting in muscle tightness, especially with rapid passive or active movement and stretch. These spasms can become powerful enough to hinder physical and occupational therapies, make it difficult to dress or clean a patient, and make transfers dangerous. Spasticity often increases in the early weeks to months following an SCI and either will remain or dissipate over time. Initial management of spasticity is via therapy and stretching; however, often this alone is not enough. Oral baclofen is the pharmacologic agent of choice for spasticity. It is usually started at a dose of 5–10 mg three to four times per day and gradually increased as tolerated. Side effects that can affect tolerability include sedation and confusion. It is not uncommon for persons with SCI to take doses of up to 40 mg at a time. Should oral baclofen not be sufficient to control the spasms, then secondary oral agents such as tizanidine can be tried [caution with orthostatic hypotension(OH)] or intrathecal baclofen via an intrathecal pump should be considered. Cancer patients with spasticity may not be medically able to undergo pump implantation, and in this case benzodiazepines or dantrolene could also be considered to treat spasm. Of note, dantrolene can lead to hepatotoxicity, and as such liver function tests should be followed closely and it should be avoided in those with liver disease.
Autonomic Dysfunction
Autonomic dysreflexia
Patients with SCI at thoracic level 6 or higher are at increased risk of autonomic dysfunction. This can present in several ways, the most serious of which is autonomic dysreflexia (AD). AD is defined as a sudden rise in systolic blood pressure (20–40 mm Hg above baseline) that is frequently associated with bradycardia. Of note, the normal range of systolic blood pressure for a person with SCI at T6 or above may be as low as 90–110 mm Hg. AD is a response to a noxious stimulus below the level of SCI, most commonly related to distal bowel (e.g., constipation) or bladder (e.g., elevated bladder pressure) irritation. It results in a reflex sympathetic response above the neurologic level of injury leading to hypertension and tachycardia. Baroreceptors are then able to regulate heart rate often leading to bradycardia in an attempt to mitigate the rapid rise in blood pressure. The body makes an attempt to trigger a parasympathetic response to counter the hypertension, but because these patients have SCI lesions at T6 or above, there is impaired parasympathetic control of the splanchnic vasculature and hypertension remains until the noxious stimulus is removed. Often the only sign of AD are a patient’s subjective complaints that often include headache, flushing, or sweating above the lesion, itchiness, or stuffy nose. AD is a potentially life-threatening condition and can result in hemorrhagic stroke. Treatment of AD primarily involves removal of the noxious stimulus. If a practitioner suspects AD, the following steps should be taken ( Fig. 10.2 ):