Chapter Outline
Glanzmann Thrombasthenia: Defective Platelet Integrin α IIb β 3
Other Integrins in Inherited Platelet Disorders
Bernard-Soulier Syndrome: Defective Glycoprotein Ib/IX Complex
Platelet-Type von Willebrand Disease (Pseudo–von Willibrand Disease)
Other Inherited Defects of Platelet Receptors
Defects in Signal Transduction
Platelets are small (1 to 4 µm in diameter) and were once thought to be fragments of other mature blood cells, dismissed as “blood dust.” They are now known to be highly specialized and organized structures released (by a still partly understood process called thrombopoiesis ) from megakaryocytes. Platelets are a critical component for the first phase of hemostasis (formation of the platelet plug), which can halt the loss of blood from vessels whose endothelial integrity has been interrupted, though it has become increasingly clear that platelets have important roles in maintaining vascular integrity and the inflammatory response. If platelets are deficient in number or defective in function, excessive bleeding may occur. The clinical manifestations of platelet-type bleeding typically involve the skin or mucous membranes and include petechiae, ecchymosis, epistaxis, menorrhagia, and gastrointestinal hemorrhage ( Box 30-1 ). Intracranial bleeding can occur, but it is infrequent. The deep-muscle hematomas and hemarthrosis typically seen in patients with defects in the fluid-phase (plasma) hemostatic system infrequently occur in platelet disorders.
Epistaxis, petechiae, purpura, ecchymosis
Gastrointestinal hemorrhage
Menorrhagia
Rarely, intracranial bleeding or hemarthrosis
Inherited platelet disorders can involve a qualitative and/or quantitative defect and are often broadly classified according to one of these two categories. In this chapter, we have classified the platelet disorders by their predominant feature, although many involve combined defects in both platelet number and function. For example, Bernard-Soulier syndrome (BSS) results from defects in the platelet receptor glycoprotein Ibα/Ibβ/IX/V (GPIb/IX) complex, which binds von Willebrand factor (VWF) and is critical for adherence of platelets at sites of vascular injury. This receptor is anchored to the cytoskeleton and is also important in platelet formation from megakaryocytes. Thus many of these patients have macrothrombocytes and low platelet counts, as well as a defect in platelet function. Furthermore, although many inherited platelet disorders have been described, most are extremely rare. Even the well-known disorders such as BSS and Glanzmann thrombasthenia (GT) are uncommon, with a frequency in the general population of 10 −5 to 10 −6 , and are mostly seen in inbred populations or consanguineous relationships. In aggregate, however, these inherited disorders are not uncommon and, given the diverse nature of the underlying defects, may pose a significant diagnostic challenge.
Qualitative Disorders
Platelet Membrane and Receptors
Sensing abnormalities in their microenvironment, adhering to damaged vascular walls, and aggregating to each other are central functions of circulating platelets. Many of the membrane receptors in these processes have been cloned, and our understanding of the interplay between membrane receptors, intracellular molecules, and the cytoskeleton continues to increase. Initially, these protein receptors were classified by their electrophoretic mobility and molecular mass and numbered sequentially. Now it is clear that many of these proteins are members of several large families of receptors, including the integrin α/β heteroduplexes, the leucine-rich receptors, the G protein–coupled receptors (GPCRs), and the immunoglobulin superfamily. Such a classification takes advantage of the similar structure and function of members of these families. Frequently, family members share a common family of ligands or activate cells by a common pathway. Table 30-1 lists the members of these families of receptors that are found on platelets, as well as several receptors that do not belong to any of these families. Many of these receptors have other historical names, and these, as well as their cluster differentiation (CD) antigen nomenclature, are noted in the table. Many of the receptors involved in inherited platelet disorders are described in the following text.
| Class of Receptor | Receptor | Other Names | Number of Receptors per Platelet | Ligand |
|---|---|---|---|---|
| Integrins | α IIb β 3 | GPIIb/IIIa, CD41b | »80,000 | Fibrinogen, VWF, fibronectin |
| α V β 3 | »500 | Vitronectin, osteopontin | ||
| α 2 β 1 | CD49b | »2000 | Collagen | |
| α 5 β 1 | CD49e | »4000 | Fibronectin | |
| α 6 β 1 | CD49f | Laminin | ||
| Leucine-rich repeats receptor | GPIb-IX | CD42a, b, c | »25,000 | VWF, thrombin, P-selectin |
| G protein–coupled receptors | PAR-1 | »2000 | Thrombin | |
| PAR-4 | Low | Thrombin | ||
| P2Y1 | ADP | |||
| P2X1 | ADP | |||
| P2Y12 | ADP | |||
| a 2A | »700 | Epinephrine | ||
| TP | »1000 | Thromboxane | ||
| IP | Prostaglandin I 2 | |||
| CXCR1 and CXCR2 | »2000 each | Interleukin-8 | ||
| CXCR4 | »2000 | Stromal-derived factor 1 | ||
| CCR4 | »2000 | CCL22 | ||
| Immunoglobulin superfamily receptors | GPVI | 1000-3000 | Collagen | |
| P-selectin | GMP-140, PADGEM, MARK | P-selectin, glycoprotein-1 | ||
| PECAM-1 | CD31 | »10,000 | PECAM-1 | |
| FcγRIIA | CD32 | 1000-5000 | Immune complexes | |
| Others | GPIV | CD36 | »25,000 | Collagen |
| p65 | Collagen |
Glanzmann Thrombasthenia: Defective Platelet Integrin α IIb β 3
In 1918, a Swiss pediatrician, Eduard Glanzmann, described a heterogeneous group of disorders that he termed thrombasthenie (weak platelets); these disorders were characterized by normal platelet counts but abnormal clot retraction. In 1956 it was noted that these platelets failed to spread onto a surface or to stick to each other (aggregate). Glanzmann thrombasthenia (GT; MIM 273800) is now known to be a rare, inherited, autosomal recessive bleeding disorder, the hallmark of which is failure of platelets to bind fibrinogen and aggregate after activation. The underlying defect is an abnormality in the genes encoding either chain of the integrin α IIb β 3 fibrinogen receptor (see Table 30-1 ). GT is the most common of the inherited platelet disorders associated with a severe bleeding phenotype.
Biology of the α IIb β 3 Receptor
The α IIb and β 3 subunits are encoded by separate genes ( ITGA2B and ITGB3 ) that are closely linked on chromosome 17q21-23. α IIb is approximately 145 kD in size and contains 18 cysteine residues arranged into 9 disulfide bonds that are rather evenly spaced throughout its length ( Fig. 30-1 ). The α IIb prochain complexes with the β 3 subunit in the endoplasmic reticulum. During maturation in the Golgi body, the α IIb prochain is cleaved into the α IIb heavy fragment and the α IIb light fragment, with the two fragments remaining linked by a disulfide bond. Like many other integrin α chains, α IIb contains four calcium-binding domains near its N-terminal. This region in all α subunits contains seven homologous repeats that fold into a β-propeller structure ; portions of the surface loops of this structure are critical for ligand binding (in interaction with the βA domain of the β 3 subunit).
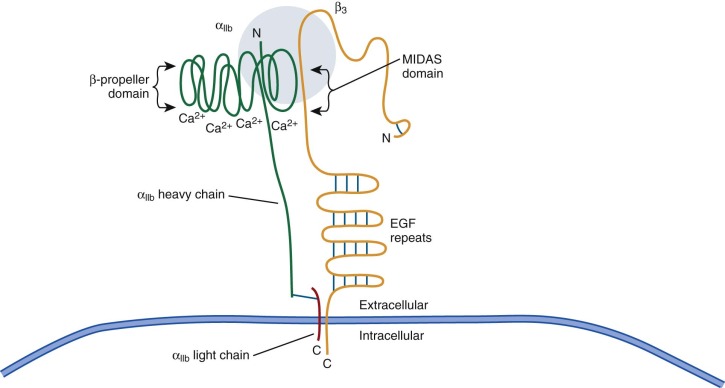
Similar to other integrin β chains, β 3 is approximately 90 kD and contains 762 amino acid residues in its mature form (see Fig. 30-1 ). β 3 contains five cysteine-rich regions for a total of 56 cysteine residues, including a large disulfide-bonded loop, termed the βA region, that extends from amino acids Cys5 to Cys435. This loop participates in fibrinogen binding and contains three divalent cation-binding domains that appear to be important in ligand binding. One of these cation-binding sites (the βA MIDAS–metal ion–dependent adhesion site) interacts directly with ligand, and binding of cations to the βA domain may stabilize the ligand-occupied conformation.
There are about 8 × 10 4 α IIb β 3 receptors per platelet, making this receptor the most abundant one on platelets (see Table 30-1 ). Most of these receptors are located on the platelet surface, although a portion are found on the inner surface of alpha granules and are involved in the localization of fibrinogen to these granules. On resting platelets, α IIb β 3 exists in a folded, inactive state that does not interact with its ligand. However, upon platelet activation, an “inside-out” signaling event takes place in which the α IIb β 3 complex is activated and unfolded like a switch blade, thereby resulting in binding of fibrinogen at the N-terminals of the two proteins and platelet aggregation. After ligand binding, an “outside-in” signal mediates integrin-cytoskeleton interactions and platelet spreading.
Many integrin ligands contain an arginine-glycine–aspartic acid (RGD) motif that participates in integrin binding. Conversely, peptides containing RGD act as competitive inhibitors of ligand binding. For example, the RGD motif located in the C1 domain of VWF appears to be necessary for binding of VWF to α IIb β 3 . In contrast, deletion of the two RGD sequences in the fibrinogen α chain does not impair its ability to bind to α IIb β 3 . Rather, binding of fibrinogen to α IIb β 3 requires a KQAGD sequence located at the carboxyl-terminal of the fibrinogen γ chain.
Whereas expression of the α IIb chain is restricted to megakaryocytes, the β 3 subunit is expressed more widely as a component of the α V β 3 (vitronectin) receptor. Mouse studies have shown that deletion of α V β 3 function results in placental defects and osteosclerosis, although to date no differences have been noted between patients with GT caused by an α IIb or β 3 defect.
Clinical Features
Thrombasthenia is characterized by repeated mucocutaneous bleeding beginning at an early age ( Fig. 30-2, A ). Epistaxis and gastrointestinal bleeding are frequent issues in early childhood that often require intervention, as well as early iron supplementation. Menorrhagia is a critical problem in teenage girls. The bleeding that normally accompanies pregnancy, surgical procedures, tooth extraction, or physical trauma can be excessive in GT patients. Unprovoked intracranial or gastrointestinal hemorrhage occurs and accounts for a significant portion of the observed 5% to 10% premature mortality rate. In addition, some patients experience joint bleeding or muscle hematomas more characteristic of the hemophilias.

Most frequent is recurrent and problematic epistaxis often complicated by prior nasal cauterizations that damage the local nasal architecture. Frequently, local compression strategies or topical applications of thrombin are useful when bleeding occurs. DDAVP (1-deamino-8-δ-arginine vasopressin) has been used as well, and oral contraceptive management of menorrhagia is generally sufficient. Platelet transfusions may be useful until resistance to platelet infusions develops, although patients do not necessarily form antibodies to the α IIb β 3 receptor. Antibodies may be particularly problematic because they may not only result in increased platelet clearance or platelet refractoriness but may also interfere with platelet function. Case reports and small case series suggest that recombinant activated factor VII may be a useful supplement to platelet transfusions. We have also successfully used embolization of arterioles feeding the nose and uterus in cases of recurrent, life-threatening hemorrhage.
Incidence
Although infrequent worldwide, GT is more prevalent among certain isolated populations and in the setting of consanguineous relationships, particularly among Arab populations, Iraqi Jews, French gypsies, and individuals from southern India. Heterozygotes for this disease have approximately 50% of the normal number of α IIb β 3 receptors, but generally no evidence of platelet dysfunction or clinically significant bleeding. Although most cases are observed in specific populations or in the setting of consanguinity and are thus homozygous for a shared mutation inherited from both parents, about one third of patients with identified mutations are compound heterozygotes.
Classification and Laboratory Diagnosis
In 1972, Jacques Caen classified GT according to platelet intracellular fibrinogen content and the ability of platelets to retract a fibrin clot. Type I patients, representing 80% of those studied, lacked platelet fibrinogen and had an absence of clot retraction. Type II thrombasthenic platelets contained appreciable levels of platelet fibrinogen and maintained some clot retraction capability. Soon thereafter, the technique of sodium dodecyl sulfate–polyacrylamide gel electrophoresis became widespread, and it became clear that there were three different patterns seen with thrombasthenic platelet membrane glycoproteins. Type I platelets lacked detectable levels of α IIb β 3 , whereas type II platelets expressed moderate (15% to 25%) levels of these glycoproteins. Adding to the complexity of this classification was the identification of variant forms of thrombasthenia characterized by normal to nearly normal levels of a dysfunctional form of α IIb β 3 (type III). Finally, a related integrin dysfunction syndrome was identified involving a GT-like platelet defect along with a white cell disorder termed leukocyte adhesion deficiency type III . These patients have a deficiency of Kindlin-3 (encoded by the FERMT3 gene on chromosome 11), an intracytoplasmic signaling molecule involved in the function of β 2 – as well as β 3 -based integrins. Patients have both GT-like bleeding manifestations and the infectious complications and leukocytosis of leukocyte adhesion deficiency.
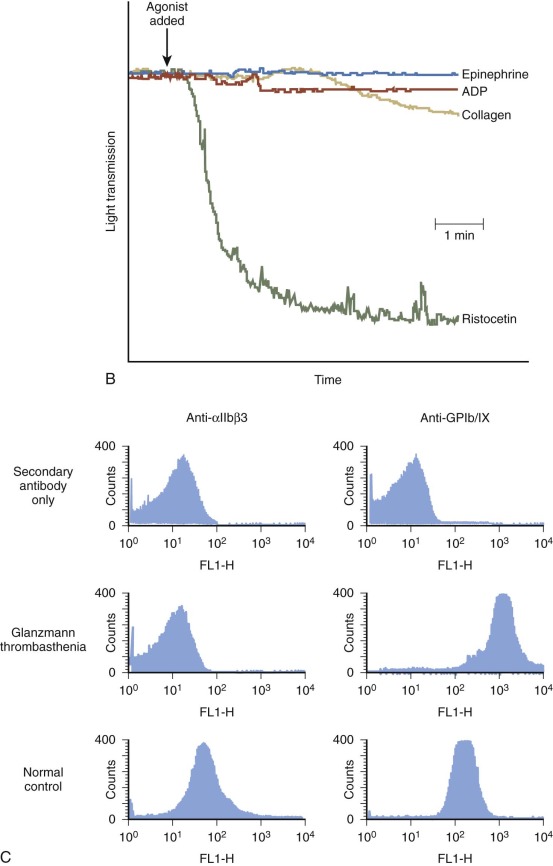
GT patients fail to aggregate in response to physiologic agonists such as adenosine diphosphate (ADP), thrombin, or epinephrine (see Fig. 30-2, B ) because they all have a functional deficiency of platelet surface α IIb β 3 receptors. The failure to bind fibrinogen and other adhesive ligands is the reason for the inability of platelets to aggregate. No correlation exists between any of the proposed subtypes of GT and the severity of bleeding symptoms in patients. Some patients with no α IIb β 3 have relatively mild clinical symptoms, whereas others with a full complement of α IIb β 3 , albeit dysfunctional, can have frequent bleeding episodes requiring repeated transfusions.
Currently, the most common method used for determining levels of α IIb β 3 on thrombasthenic platelets involves flow cytometry and immunoblot analysis. Figure 30-2, C illustrates flow cytometric analysis of the α IIb β 3 content of both a normal control and a GT patient with a severe deficiency of α IIb β 3 secondary to a mutation in one of the Ca 2+ -binding domains of her α IIb chain. This mutation blocks export of the α IIb β 3 receptor out of the endoplasmic reticulum and into the Golgi body for final processing, as indicated by failure to cleave the α IIb prochain into an α IIb heavy chain.
Several hundred individuals with GT have been described in the literature. To date, more than four hundred different mutations have been described. As in most genetic disorders, the molecular abnormalities have been found to range from major deletions and inversions to single point mutations. Some of the better-characterized subgroups of mutations are described in the following text.
The earliest and largest group of thrombasthenic mutations described failed to express α IIb β 3 on the cell surface. Several of these patients had major deletions or inversions in their α IIb or β 3 genes. In addition, a number of point mutations or small deletions in the α IIb or β 3 genes have been described with no surface expression of α IIb β 3 ; both chains are formed intracellularly and may assemble the complex but fail to undergo intracellular processing or reach the cell surface. These defects result in the type 1 form of thrombasthenia. One major subgroup of these patients involves missense mutations in the N-terminal β-propeller repeats of α IIb near the proposed Ca 2+ -binding sites on its lower surface as exemplified by the patient in Figure 30-2 .
Another group of mutations occur in the same β-propeller region of α IIb but take place on the upper surface and have expressed surface receptor that cannot bind its ligand. These mutations are located within three upper loops of the β-propeller, the connecting loop between the second and third blades, the loop in the middle of the third blade, and the loop connecting the third and fourth blades. These mutations occur within the pocket of α IIb that binds RGD ligand.
Other thrombasthenic patients with significant levels of nonfunctional α IIb β 3 surface expression have mutations that involve the β 3 chain. These mutations result in a surface α IIb β 3 that is easily dissociable by chelation of external calcium ions. Many of these missense mutations have been identified within the cation-binding MIDAS domain. The importance of these sites was reinforced by the identification of a group of in vitro–generated mutant α IIb β 3 receptors expressed in Chinese hamster ovary cells, which provided independent support for the importance of the MIDAS domain in ligand binding.
A second group of dysfunctional mutations with normal levels of surface α IIb β 3 receptors are localized to the cytoplasmic domain of β 3 , thus demonstrating the importance of this domain for integrin activation and regulation of ligand binding. These mutations do not affect surface expression of platelet α IIb β 3 complexes, but mutant receptors are unresponsive to agonist stimulation. These mutations provide compelling evidence for the role of the β 3 cytoplasmic tail in downstream platelet activation by α IIb β 3 .
Other Integrins in Inherited Platelet Disorders
Other integrins that are infrequently associated with platelet dysfunction include the α 2 β 1 (glycoprotein Ia/IIa) receptor, an Mg 2+ -dependent collagen receptor on many cells, including platelets. In platelets, collagen not only serves as an adhesive substrate but it also functions as an agonist for platelet aggregation. Patients with histories of bleeding have been reported whose platelets failed to respond normally to collagen and lacked α 2 β 1 , thus suggesting that binding of collagen to α 2 β 1 is a necessary component for collagen-induced signaling. In addition, another report described several families with autosomal dominant thrombocytopenia and mild platelet dysfunction associated with mutations in the α 2 subunit.
There is substantial variability in the density of α 2 β 1 on the surface of platelets from different individuals. These differences in α 2 β 1 expression are associated with the inheritance of three different α 2 alleles. The higher the receptor density, the greater the attachment of platelets to type I collagen. Some, but not all, epidemiologic surveys suggest that increased α 2 β 1 density is a risk factor for cardiovascular disease. An increased prevalence of the allele with lower α 2 β 1 density has been reported in patients with type 1 von Willebrand disease (VWD), which suggests that this allele may contribute to bleeding symptoms in these patients.
Bernard-Soulier Syndrome: Defective Glycoprotein Ib/IX Complex
Bernard-Soulier syndrome (BSS: MIM 231200) was first described in 1948 in a 5-month-old infant with a prolonged bleeding time and giant platelets on blood smear and who had had a sibling who died of hemorrhage. Over the following years, additional patients with the combination of mucocutaneous bleeding; enlarged platelets; normal platelet aggregation with ADP, collagen, and epinephrine with a delayed response to thrombin; and absent platelet aggregation with human VWF and ristocetin or with bovine VWF alone were described as having BSS, now the second most recognized inherited severe platelet disorder.
Biology of the Glycoprotein Ib/IX Complex
Adhesion through the GPIb/IX complex involves binding of VWF to the subendothelium. Platelet membranes contain two binding sites for VWF (see Table 30-1 ). One of these sites requires previous platelet activation and is located on the platelet membrane α IIb β 3 complex. The second binding site involves the GPIb/IX complex, and it is this membrane complex that is crucial for initial attachment and proper adhesion to the extracellular matrix of a damaged vessel wall. It should be pointed out that the GPIb/IX complex may also bind to other ligands, including P-selectin, thrombospondin-1, high-molecular-weight kininogen, and Mac-1. Furthermore, GPIb/IX also binds thrombin, and its role in the activation of platelets by thrombin is discussed later.
The GPIb/IX complex is the second most abundant receptor on the platelet membrane surface, with approximately 25,000 copies per platelet. This complex actually consists of four different proteins ( Fig. 30-3 ), all of which have one or more leucine-rich repeats composed of a 24–amino acid motif with 7 conserved leucine residues. Other proteins have been described with this leucine-rich repeat, and these regions mediate ligand binding.
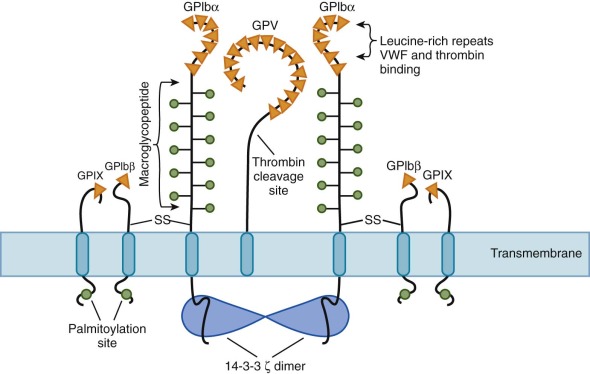
GPIbα is the largest subunit (135 kD, 610 amino acids, chromosome 17p12) and has 7 leucine repeats. It is susceptible to cleavage by trypsin or calpain, which gives rise to a heavily glycosylated 135-kD fragment known as glycocalicin . In addition to containing the binding site for VWF, the glycocalicin portion of GPIbα also binds thrombin. Under normal flow in vivo, plasma VWF does not bind to the GPIb complex, but under shear stress conditions, VWF simultaneously binds to collagen and the GPIb/IX complex. In clinical assays, the antibiotic ristocetin or the venom-derived protein botrocetin is used to mimic this effect by inducing conformational changes in VWF that promote binding to GPIb/IX in stirred platelet-rich plasma.
GPIbα is disulfide-bonded to GPIbβ (25 kD, 181 amino acids, chromosome 22q11.2) through a single cysteine residue located in each subunit near the transmembrane domains of GPIbα and GPIbβ (see Fig. 30-3 ). This peptide has only one leucine repeat. The cytoplasmic tail of GPIbβ contains a filamin-binding site that links the receptor complex to F-actin below the membrane and a binding site for 14-3-3ζ. The disulfide-bound GPIbα-β is noncovalently associated with platelet GPIX and GPV. GPIX is the smallest member of the GPIb complex (22 kD, 160 amino acids, chromosome 3q29), with only one leucine repeat. GPV (82 kD, 344 amino acids, chromosome 3q21) is a transmembrane protein with 15 leucine repeats. This protein is a proteolytic substrate for thrombin and releases a 69-kD soluble fragment. GPV is present in only a single copy per complex, whereas there are two copies of the other three subunits in a single receptor (see Fig. 30-3 ). Furthermore, cleavage of the GPV extracellular domain by thrombin bound to GPIbα activates platelets. Consistent with this observation, the GPV-knockout mouse has a mild prothrombotic state.
Binding of activated VWF to the GPIb/IX complex activates platelets through activation of phospholipase C (PLC) and mobilization of protein kinase C, which, together with increases in [Ca 2+ ] i , promotes platelet secretion and potentiates platelet aggregation. The GPIb-IX complex also appears to activate the cell by binding to the cytoskeleton protein 14-3-3ζ and by interacting with the FcγRIIA receptor on platelets, where it activates an intracellular tyrosine–based activation motif receptor (see Fig. 30-3 ).
Clinical Features
The bleeding manifestations of patients with BSS are similar to those of other patients with severe platelet dysfunction and center on mucocutaneous bleeding with purpura, epistaxis, gastrointestinal hemorrhage, and menorrhagia. Alloantibodies to components of the GPIb/IX complex can develop after platelet transfusions with resulting platelet refractoriness. Care of bleeding in these patients is similar to that of patients with GT discussed earlier, including the use of DDAVP. Studies evaluating DDAVP have demonstrated improved bleeding times, although the improvement did not correlate with the ability of DDAVP to increase the level of circulating VWF. Recombinant activated factor VII has also been reported to be useful in patients with BSS, but data are still very limited and it is probably most effective in combination with platelet transfusion.
Classification and Laboratory Diagnosis
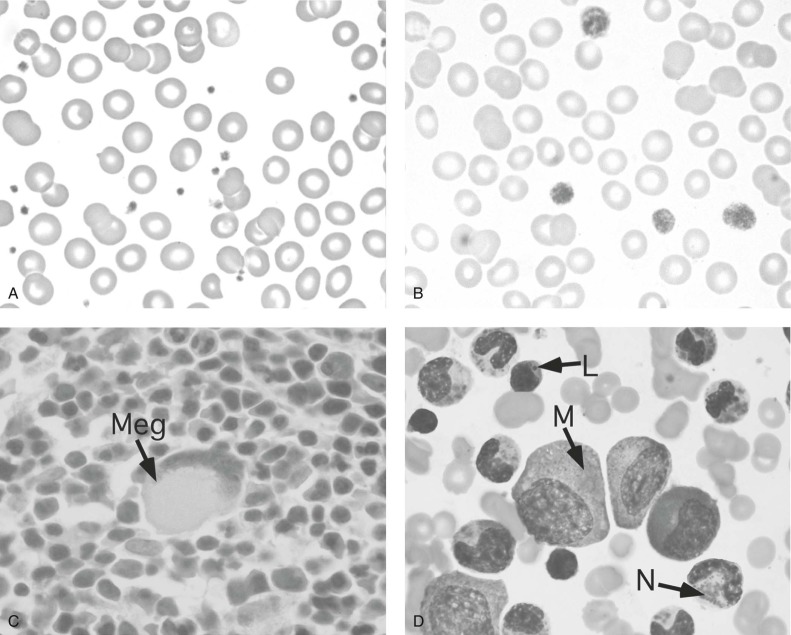
BSS patients have a variable degree of thrombocytopenia. Most patients are thrombocytopenic to some extent, but some patients may have platelet counts as low as 20,000/µL. However, care must be taken to avoid underestimation of platelet number due to the macrothombocytopenia. The platelets have a mean diameter ranging from 3 to 20 times normal ( Figs. 30-4, B and 30-5, A ). Other cell types appear normal. In general, the circulating total platelet mass in people is more precisely conserved than the platelet count is, and part of the decrease in platelet count in BSS may well reflect this compensatory mechanism. Megakaryocytes in this disorder appear normal in size and appearance by light microscopy. However, on electron microscopy, a striking feature in these cells is the variable and intermittent nature of the demarcation system, which is often vacuolar. Absence of GPIbα results in an abnormal demarcation membrane in a mouse model of BSS. Studies have shown that GPIb/IX contributes to platelet formation in that this complex lines up with the cytoskeleton during thrombopoiesis and plays a role in cell cycle regulation.
Bleeding times and platelet functional analyzer–100 measurements are prolonged in these patients, but the distinctive abnormality of BSS platelets is the failure of agglutination in the presence of ristocetin, an abnormality that cannot be corrected by the addition of normal plasma. Aggregation by other agonists such as ADP, collagen, and epinephrine is normal, although the response to low-dose thrombin may be delayed. Flow cytometric analysis of platelet glycoproteins shows decreased surface expression of the GPIb/IX complex and may aid in the diagnosis.
Molecular Abnormalities
Molecularly, there appear to be two forms of BSS. In the biallelic form, most often homozygous mutations result in severely reduced or absent GPIb/IX expression. Heterozygous family members are usually asymptomatic with normal platelet counts. For the biallelic form, more than 50 mutations of the GP1BA, GPIBB, and GP9 genes have been defined. Few patients are compound heterozygotes, suggesting that this is a rare disease outside of specific populations. Most of the mutations described are missense or nonsense mutations. Deletions are uncommon but do occur, as in one patient who had partial deletion of the GPIBB gene on chromosome 22q11.2 in association with velocardiofacial syndrome. In the monoallelic form, the platelet count is usually somewhat decreased and the bleeding tendency is often less dramatic. However, in some families, there is significant thrombocytopenia and severe bleeding, in particular with the p.Ala156Val mutation in Italy, and three other missense mutations (p.Asn57His, p.Tyr70Asp, and p.Leu73Phe) in GP1BA . These mutations are associated with decreased expression of the GPIb/IX complex and macrothrombocytopenia. It is not clear why some mutations appear to exhibit a dominant negative effect so that heterozygous patients manifest disease whereas other heterozygous patients are asymptomatic. Indeed, patients with 22q deletion syndrome (22qDS, velocardiofacial syndrome, DiGeorge syndrome) often have deletion of one copy of GP1BB but may only manifest mild macrothrombocytopenia with decreased expression of the GPIb/IX complex.
Platelet-Type von Willebrand Disease (Pseudo–von Willibrand Disease)
Platelet-type von Willebrand disease (VWD), also known as pseudo–von WIllibrand disease , is an autosomal dominant bleeding disorder often associated with a prolonged bleeding time, mild thrombocytopenia, and decreased circulating levels of high-molecular-weight VWF multimers. Patients with this disorder have a mild to moderate bleeding diathesis. Platelet-type VWD is very similar to type 2B VWD (see Chapter 31 ) in that both are characterized by platelet agglutination in the presence of low concentrations of ristocetin and by decreased circulating levels of high-molecular-weight VWF multimers. Binding of VWF to circulating platelets results in the reduced levels of VWF seen in these patients, as well as some degree of platelet activation, mild or intermittent thrombocytopenia, and an increased risk of clotting.
Unlike type 2B VWD, where mutations in VWF result in increased affinity for a normal platelet GPIb/IX complex, pseudo-VWD is caused by an alteration in the platelet GPIb/IX complex that leads to increased affinity for normal VWF multimers. Platelet-type VWD can be distinguished from type 2B VWD by the addition of normal VWF to patient platelet-rich plasma, which results in spontaneous aggregation of pseudo-VWD platelets but not type 2B VWD platelets. It is important to distinguish patients with platelet-type VWD from other VWD patients because care of patients with VWD often involves infusion of VWF that contains factor VIII concentrates or DDAVP. This therapy can worsen the thrombocytopenia in patients with platelet-type VWD. A patient with mild thrombocytopenia and a prolonged bleeding time should be evaluated for this disorder.
The molecular basis of platelet-type VWD has been defined in multiple families. Mutations, such as Gly233Val and Met239Val, occur in the region of GP1BA that has been previously shown to be important in VWF binding. It has been hypothesized that these mutations alter GPIbα such that it maintains an adhesive conformation much as wild-type GPIb/IX receptor becomes activated on exposure to components of the extracellular matrix at sites of vascular injury and flow.
Montreal platelet syndrome , characterized by macrothrombocytes, a prolonged bleeding time, spontaneous aggregation of platelets at pH 7.4, and a defect in platelet calpain metabolism, was thought to be a distinct macrothrombocytopenia, but in 2009 was shown to be a particular subtype of type 2B VWD, with a V1316M mutation in VWF . This disorder demonstrates the overlap between the described phenotypes in these inherited platelet disorders and the value of molecular diagnosis.
Other Inherited Defects of Platelet Receptors
G Protein–Coupled Receptors
Many agonists that activate platelets, such as thrombin, ADP, and thromboxane A 2 (TxA 2 ), activate cells through a G protein–coupled receptor (GPCR) (see Table 30-1 ). These seven-transmembrane receptors are linked to a heterotriplex of intracellular guanosine diphosphate (GDP)/guanosine triphosphate (GTP)–binding proteins, Gα, β, and γ. The strongest platelet agonist is thrombin. As mentioned previously, one receptor for thrombin is the GPIb/IX complex. However, thrombin appears to have its greatest effect on human platelets through two GPCRs, protease-activated receptor 1 (PAR-1) and PAR-4, with PAR-1 being the major human receptor. Thrombin binds to PAR-1, releases an N-terminal peptide, and leaves a new N-terminus. An oligopeptide of this N-terminal sequence, SFLLRN, can mimic the effects of thrombin and activate platelets. Surprisingly, no patients with defects in PAR-1 have been described. In mice, PAR-1 is not important for platelet biology, although its absence does have consequences because it is expressed on other tissues. Instead, on murine platelets, PAR-3 and PAR-4 are biologically relevant.
ADP is stored in platelet-dense granules and released upon platelet activation. When added to platelets in vitro, ADP causes TxA 2 formation; protein phosphorylation; and increase in cytosolic Ca 2+ shape change, aggregation, and secretion. ADP also inhibits the formation of cyclic adenosine monophosphate (cAMP). Several patients with bleeding defects have been described whose platelets showed greatly diminished responsiveness to ADP and reduced numbers of binding sites for ADP analogues, a phenotype not dissimilar from that produced by ADP antagonists such as ticlopidine or clopidogrel. ADP receptors on human platelets include P2Y1, P2Y12, and P2X1. P2Y1 is a GPCR that activates PLC. P2Y12 inhibits cAMP formation by adenyl cyclase. P2X1 is a ligand-gated nonspecific cation channel that may contribute to the influx of extracellular Ca 2+ that occurs in response to ADP. Mutations in each of these receptors have been reported in patients and have resulted in mild to moderate bleeding symptoms.
In several respects, epinephrine is unique among human platelet agonists in that it causes aggregation and secretion but does not cause the cytoskeletal reorganization that underlies shape change. Activation of PLC by epinephrine appears to be dependent on TxA 2 formation and can be suppressed with aspirin. Aspirin also blocks second-wave, or secretion-dependent, platelet aggregation in response to epinephrine. This gives rise to a characteristic aggregometer tracing in which epinephrine-induced primary aggregation is followed by disaggregation of the platelet clumps. Platelet responses to epinephrine are mediated by α 2 -adrenergic receptors. There are reports of families in which a mild bleeding disorder was associated with impaired epinephrine-induced aggregation and reduced numbers of α 2 -adrenergic receptors. However, platelets from up to 10% of apparently normal people may fail to aggregate when challenged with epinephrine, and the clinical significance of this finding is unclear.
TxA 2 is produced from arachidonate in platelets by the aspirin-sensitive cyclooxygenase pathway. When the stable thromboxane analogue U46619 is added to platelets in vitro, the platelets undergo shape change, aggregation, secretion, phosphoinositide hydrolysis, protein phosphorylation, and an increase in [Ca 2+ ] i . Similar responses are seen when platelets are incubated with exogenous arachidonate. Platelets express a GPCR for TxA 2 . Platelets also contain a receptor for prostacyclin (PGI 2 ), an endothelial cell prostaglandin that is a potent platelet inhibitor. Binding of PGI 2 to this receptor stimulates adenylate cyclase activity and inhibits platelet function. A few patients have been described who have a mild bleeding disorder caused by congenital absence of the TxA 2 receptor. Two Japanese patients have had their mutations defined and were found to have an R60L substitution in the first cytoplasmic loop of TXA2R . In addition, three patients have been described who have normal TxA 2 binding but absent platelet aggregation in the presence of this agonist, thus suggesting uncoupling between the receptor and downstream signaling events. There are as yet no known clinical disorders ascribed to the PGI 2 receptor, but deletion of the gene in mice increases their risk for thrombosis after arterial injury and polymorphisms in the PGI 2 gene ( PTGIS ) have been reported to be risk factors for cerebrovascular disease.
Immunoglobulin Superfamily Receptors
GPVI, a 62-kD protein encoded by a gene ( GP6 ) on human chromosome 19q13.4, is a member of the immunoglobulin superfamily of receptors and is the platelet-signaling receptor for collagen. Unlike α 2 β 1 , GPVI’s interaction with collagen initiates intracellular signaling via its surface partner, the FcRγ chain. Signaling occurs via phosphorylation of PLCγ2 and Syk. Fab antibodies derived from GPVI-deficient individuals who had received platelet transfusions recognize GPVI and are capable of blocking activation of platelets by collagen, thereby resulting in thrombocytopenia and internalization and/or cleavage of surface GPVI. Convulxin, a snake venom protein isolated from a South American rattlesnake, is also a potent activator of platelets via GPVI.
GPVI seems to be the major collagen receptor on platelets, although a few patients have been described who lack GPVI and show only a mild bleeding tendency. A mutation in the GP6 gene has been linked to absence of collagen-induced platelet activation. These patients have some residual platelet aggregation to collagen, which is likely to be through the α 2 β 1 receptor. In addition, autoantibodies to GPVI have been described in patients with immune thrombocytopenic purpura and systemic lupus erythematosus. These patients lack surface GPVI on their platelets and show a mild bleeding diathesis with abnormal platelet aggregation to collagen. Interestingly, several genetic polymorphisms affecting GPVI surface expression have been described and suggested to be a risk factor for myocardial infarction and may be associated with increased risk of pregnancy loss. Platelets with less GPVI showed less vigorous aggregation in vitro, although further study is ongoing on the true relevance of these findings.
Scott Syndrome
The platelet surface functions as one of the principal sites for plasma coagulation reactions by providing a surface on which coagulation factor complexes assemble and accelerate these important reactions several thousand-fold. This property of platelet membranes has been termed platelet factor 3 activity . In a resting platelet, there is little anionic phospholipid on the platelet’s surface. Activation by agonists such as thrombin or collagen is thought to reorient membrane phospholipids and bring anionic phospholipids, chiefly phosphatidylserine, to the outer leaflet. Activation also induces membrane vesiculation and the production of platelet microparticles with procoagulant activity.
Deficiency in platelet coagulant activity is a rare clinical event that has been termed Scott syndrome, named after the propositus of a well-studied family. The patient had prolonged bleeding after dental extractions, bleeding after surgical procedures, and a spontaneous retroperitoneal hematoma. The patient secreted a normal quantity of factor V, a platelet alpha-granule constituent, but had only 25% of the normal number of binding sites for factor Xa on activated platelets. When her platelets were challenged with such agonists as thrombin and collagen, aggregation and secretion were normal but fewer anionic phospholipid-binding sites were translocated to the platelet surface. In addition, the formation of microparticles is defective in patients with Scott syndrome. Standard screening tests for plasma coagulation, such as the prothrombin time and partial thromboplastin time, which use artificial lipid micelles, are normal. Recently, a splice-acceptor site mutation in the gene encoding transmembrane protein 16F ( ANO6 ) was reported in the original proband, causing premature termination of a Ca 2+ -activated chloride channel. Transfection studies provided evidence for the essential role of transmembrane protein 16F in Ca 2+ -dependent exposure of phosphatidylserine at the platelet surface.
The diagnostic abnormality is a shortened serum prothrombin time as a result of reduced consumption of prothrombin. Additionally, these patients have a prolonged dilute Russell viper venom time that corrects when the patient’s plasma is added to normal platelets but not when normal plasma is added to the patient’s blood. This is due to the inability to generate normal platelet procoagulant activity. Patients can be readily treated with platelet transfusions, which improve hemostasis and reduce postoperative blood loss.
Defects in Signal Transduction
With multiple overlapping pathways of platelet activation, it has been difficult to define specific defects in patients with inherited disorders of platelet signal transduction. Nonetheless, a small number of patients have been described with phenotypes and abnormal platelet aggregation patterns consistent with such a diagnosis ; the number of these patients will likely increase as the diagnostic armamentarium improves because defects in these pathways may be quite common.
Abnormalities of the G proteins (Giα1 and Gαq) have been described in patients with defects in platelet aggregation to ADP and to multiple agonists including ADP, thrombin, and TxA 2 , respectively. These patients had lifelong mucocutaneous bleeding associated with these platelet defects. Both of these proteins play an important role in outside-in signaling. Mutations in Gsα (linked to receptors for such agents as PGI 2 ) have been associated with hypofunction and hyperfunction syndromes. Mutations in prostaglandin E 1 and pituitary adenyl cyclase–activating peptide (PACAP) lead to increased cAMP production, and patients with partial trisomy of 18p have increased PACAP, very increased cAMP, and severe neurologic abnormalities with a pronounced bleeding tendency and mild thrombocytopenia. These patients (signaling defect patients) may be found to have abnormal clot retraction or abnormal spreading of platelets on surface-bound fibrinogen.
Another group of patients lack the ability to liberate arachidonic acid, which may be due to deficiency of phospholipase A 2 . Other patients have diminished cyclooxygenase enzyme activity that mimics an aspirin-like defect. These patients have abnormal platelet aggregation in response to ADP, epinephrine, collagen, and arachidonic acid but respond normally to prostaglandin G 2 , consistent with a defect in cyclooxygenase-1 and subsequent TxA 2 synthesis. Most patients with this disorder have normal antigen levels of a functionally abnormal prostaglandin synthetase. Other patients have been described who have decreased protein expression. Additionally, patients have been reported who have mutations in the gene coding for thromboxane synthetase that converts prostaglandin H 2 to TxA 2 .
Finally, pleiotropic aggregation abnormalities due to multiple pathway defects have been associated with mutations in transcription factors. Decreased platelet levels of protein kinase C and PLC-β2 have been found in patients with RUNX1 mutations (see section on FPD-AML, below), resulting in defects in aggregation and secretion as well as plekstrin phosphorylation in response to thrombin. In patients with GATA1 mutations (see below), abnormal alpha-granule biogenesis, decreased collagen-induced platelet aggregation, and abnormal GPVI-dependent tyrosine phosphorylation have been reported. Over the past few years, other models of platelet dysfunction secondary to a signaling defect have been recognized, including Wiskott-Aldrich syndrome (WAS), which is described later under disorders of platelet size and number. For all these signaling disorders, platelet transfusions are effective in the management of bleeding.
Platelet Storage Granule Defects
Human platelets contain several types of intracellular granules that can be distinguished by electron microscopy, including dense granules, alpha-granules, and lysosomes. Dense granules are the most rapidly secreted granules after platelet activation and contain ADP, adenosine triphosphate (ATP), serotonin, and calcium. Two thirds of platelet adenine nucleotides are stored in these granules, with an ADP/ATP ratio of 3 : 2, as opposed to 1 : 8 in the platelet cytoplasm. Platelet alpha granules contribute both prothrombotic and antithrombotic proteins as well as proangiogenic and antiangiogeneic proteins. After platelet activation, the alpha granules centralize in the platelet and release their contents via membrane fusion with the open canalicular system. Not all proteins present in alpha granules have the same origin. Some, such as VWF, thrombospondin, and platelet factor 4 (PF4), are synthesized in and packaged by megakaryocytes, whereas others, such as albumin, immunoglobulins, and fibrinogen, are endocytosed from plasma.
Dense Granule Defects
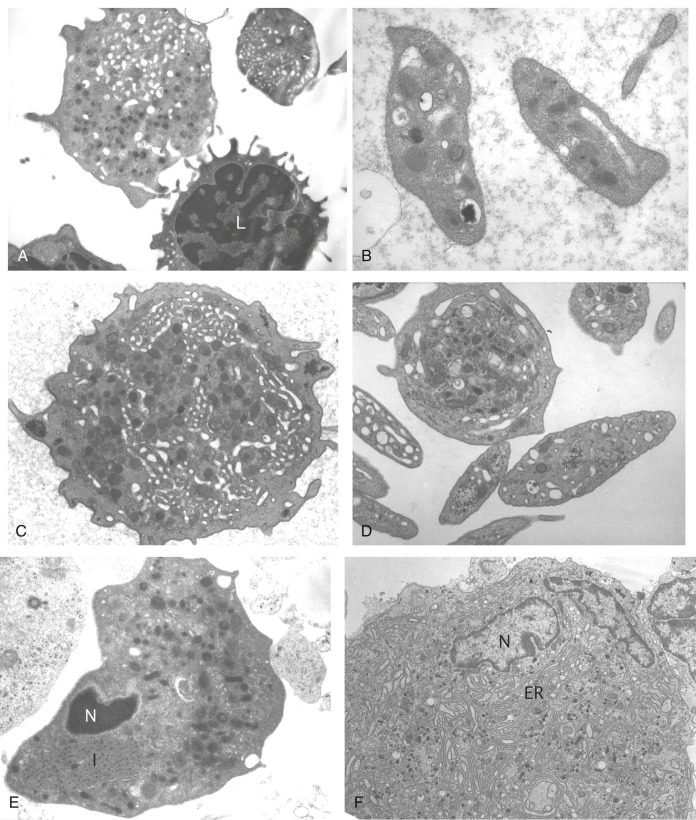
Patients with defects in platelet dense granules have a form of storage pool deficiency (δ-SPD). Patients with δ-SPD exhibit a mild to moderate bleeding diathesis, as well as abnormalities in platelet aggregation. The second wave of aggregation is absent in response to ADP and epinephrine and virtually absent in response to collagen. Bleeding times in these patients are often prolonged. In 1969 it was shown that these patients have a deficiency in total platelet ADP, and this deficit was subsequently localized to the ADP pool of the dense granule. The abnormalities in the ultrastructure of the dense granules are heterogeneous, but most patients show a characteristic lack of dense granules by electron microscopy (see Fig. 30-5 ). One study has suggested a deficiency of multidrug-resistant protein 4 may be important in two patients with δ-SPD, although the molecular basis of this defect has yet to be resolved.
The two most common SPDs are Hermansky-Pudlak syndrome (HPS: MIM 203300) and Chédiak-Higashi syndrome (CHS: MIM 214500). HPS is characterized by severe tyrosinase-positive, oculocutaneous albinism in association with photophobia, rotatory nystagmus, loss of visual acuity, excessive accumulation of ceroid-like material in reticuloendothelial cells, and a mild to moderate hemorrhagic diathesis. This disease is inherited as an autosomal recessive disorder, and although it occurs in many populations, it is seen with high frequency in people from the northwest part of Puerto Rico. Patients exhibit bruising and epistaxis in early childhood. Fatal bleeding is rare, but a significant proportion of patients will have received packed red cell or platelet transfusions in their lifetime. The major cause of death in some genotypes is progressive pulmonary fibrosis, which generally begins in the late 20s or early 30s and may be related to higher plasma serotonin levels but also clearly involves defects in the pulmonary alveolar epithelium. This disease is also associated with a 15% incidence of granulomatous colitis. To date, mutations in nine different genes ( HPS-1 to HPS-9 ) have been described in patients with phenotypic HPS, all of which encode for proteins, which are involved in the biogenesis of lysosome-related organelles such as melanosomes in melanocytes and delta granules in platelets. All known HPS proteins are components of one of four protein complexes: biogenesis of lysosome-related organelles complex 1 (BLOC-1), BLOC-2, BLOC-3, or adaptor protein complex 3 (AP-3). Some genotype-phenotype associations have also been documented. For example, pulmonary fibrosis develops in patients with mutations in HPS-1 and HPS-4 , whereas this life-threatening complication does not develop in patients with HPS-3 , HSP-5 , and HSP-6 mutations. In addition, patients with HPS-2 mutations have associated neutropenia and a predilection for infections, particularly in childhood.
CHS (MIM 214500) is a rare autosomal disorder that has been described in approximately 200 affected individuals. It is characterized by immune dysfunction, platelet storage pool defects, and partial albinism. Patients often have a white forelock or ashen hair secondary to abnormally large melanosomes. Hematologic manifestations of this disease are characterized by the presence of large intracytoplasmic granules in leukocytes, lymphocytes, monocytes, and platelets (see Fig. 30-4, D ), with a concurrent defect in mobilization of the marrow leukocyte pool, defective chemotaxis, and decreased bactericidal activity. Many other tissues also contain abnormal granules. CHS patients often die in the first 2 decades of life of either overwhelming infections or an unusual lymphoproliferative disorder called the accelerated phase , which involves fever, anemia, neutropenia, and, occasionally, thrombocytopenia, hepatosplenomegaly, lymphadenopathy, and jaundice. The latter disorder occurs in 85% of patients. Patients who survive to adulthood have progressive neurologic dysfunction. These patients are particularly susceptible to Epstein-Barr virus infections. The gene involved in CHS (LYST) is located on chromosome 1q42.1-42.2 and encodes a 3801–amino acid protein called LYST (lysosomal trafficking regulator). This complex protein is a member of the BEACH protein family and contains several ARM/HEAT domains and a BEACH motif. Mutations have been defined in an increasing number of affected patients and involve truncated products, although there is no correlation between residual protein length and clinical phenotype.
Finally, Griscelli syndrome (MIM 214450) is a rare disease in which patients have partial albinism, silver hair, and neurologic defects and/or lymphohistocytosis with associated delta-granule deficiency. Major bleeding is rare in this syndrome. Patients have mutations in myosin Va, Rab27a, or melanophilin. One patient with a mutation in RAB27A and AP3B1 showed a bleeding phenotype caused by impaired secretion, dependent platelet aggregation, and abnormal delta granules.
Whole-mount electron microscopy is often used for the diagnosis of these disorders but is complicated by the fact that dense granule numbers may be low in normal platelets. Some laboratories use serotonin release assays and assays examining ATP : ADP ratios to aid in the diagnosis of these disorders. Some studies suggest that flow cytometry on mepacrine-loaded platelets may aid in the diagnosis because mepacrine is selectively taken up by dense granules.
Alpha-Granule Defects
Patients with alpha-granule deficiencies have a bleeding phenotype similar to that of patients with dense-granule deficiencies, with mild to moderate bleeding, mild thrombocytopenia, and a prolonged bleeding time. Because of the initial light microscopic observation of gray platelets in the peripheral blood smear of these patients after Romanovsky staining, this disorder was termed gray platelet syndrome (GPS: MIM 139090). On electron microscopy, the gray platelets are most notable for the absence or marked reduction of alpha granules (see Fig. 30-5, D ). These platelets are also deficient in alpha granule–specific proteins such as PF4, VWF, factor V, and fibronectin. Gray platelet aggregation to various agonists, especially thrombin, is impaired. These platelets have a delayed and blunted Ca 2+ -mobilization response. The vacuolar structures seen in gray platelets appear to be aborted alpha granules that contain both P-selectin and α IIb β 3 in their membranes. They also contain a significant amount of proteins, including immunoglobulin and albumin, which are normally endocytosed into alpha granules. Endogenously synthesized alpha granule–specific proteins such as PF4 leak out. This latter phenomenon may contribute to the observed myelofibrosis and pulmonary fibrosis associated with this disease and may also contribute to thrombocytopenia by negative feedback on megakaryopoiesis. In 2011, several groups using various approaches simultaneously reported that mutations in NBEAL2 are responsible for GPS; the approaches included linkage analysis followed by DNA sequencing, whole-exome sequencing, and RNA-sequencing technologies. NBEAL2 belongs to a family of proteins containing BEACH domains; the proteins are highly conserved and important for protein-protein interactions, membrane dynamics, and vesicle trafficking. These diagnostic studies demonstrate the power of these technologies to identify molecular causes of inherited disorders of platelets in rare diseases and offer the possibility that in the future, underlying defects in such affected individuals will be resolved; this would not only enhance the understanding of platelet biology but would provide knowledge about an underlying platelet defect for many individuals.
In arthrogryposis, renal dysfunction, cholestasis syndrome (ARC: MIM 208085, 608552, 613401), patients also have absent alpha granules as a result of a defect in granule biosynthesis due to mutations in VPS33B or VPS16B (VIPAR), a VPS33B binding protein on chromosome 14 (C14orf133). VPS33B is a vesicle trafficking protein, a member of the Sec1/Munc18 family of proteins, and is predicted to function in vesicle docking and fusion during alpha-granule development. Patients present with neonatal cholestatic jaundice, renal tubular acidosis, and arthrogryposis with severe failure to thrive.
Other Granular Defects
A small number of patients have been described with combined alpha-granule and dense-granule deficiency . In these patients, the dense-granule deficiency is often much more severe than the alpha-granule deficiency. Patients also have mild to moderate bleeding histories, and laboratory results are similar to those of dense-granule–deficient patients, with a decreased platelet ADP\:/ATP ratio in the cells and low levels of serotonin. In one patient with a severe deficiency in both alpha granules and dense granules, there was a deficiency in total platelet P-selectin; this finding suggests that in this disorder, unlike in GPS, in which there may be an alpha-granule–targeting defect, platelet granule formation itself may be defective.
Quebec platelet syndrome is a rare disorder that results from the degradation of alpha-granule contents within the platelet because of ectopic urokinase-type plasminogen activator expression in developing megakaryocytes and its storage in alpha granules. This disorder, inherited in an autosomal dominant pattern within two large kindreds in Quebec, was previously known as factor V Quebec syndrome because the initial observations in these patients revealed a relative intraplatelet deficiency of factor V. These patients manifest moderate to severe delayed bleeding after surgery or trauma. Bleeding can be controlled by antifibrinolytic therapy with tranexamic acid, but platelet transfusions are generally ineffective. The molecular abnormality responsible for this disorder was recently found to be a direct tandem duplication of a 78-kb genomic fragment that includes PLAU (the gene encoding urokinase-type plasminogen activator) present in Quebec platelet syndrome patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree