Platelet membrane glycoproteins play a key role in hemostasis and thrombosis. Although disorders of platelet membrane glycoproteins are rare, their effects on the lives of those affected are very important. Severe deficiencies manifest themselves early during childhood with mucocutaneous bleeding. Mild deficiencies may not be diagnosed until adulthood or until the hemostatic system is stressed by surgery or trauma. The diagnosis of these disorders requires detailed laboratory investigation. Management of bleeding in patients with inherited platelet disorders requires both preventive measures and the treatment of individual bleeding episodes according to severity. The study of platelet membrane disorders also has yielded important insights into the functions of affected proteins, information that has produced some of the most successful antithrombotic drugs currently in use.
Key points
- •
Platelet membrane glycoproteins play key roles in various aspects of platelets functions and their deficiency can produce bleeding.
- •
Bleeding can be severe and manifest during childhood, or mild and only manifested in adulthood after trauma or surgery.
- •
These disorders can mimic acquired disorders and require careful histories and detailed laboratory evaluation. Management requires both preventive measures and treatment of specific bleeding episodes according to severity.
- •
The study of platelet membrane disorders also has yielded important insights into the functions of affected proteins, information that has produced some of the most successful antithrombotic drugs currently in use.
Inherited platelet disorders are rare, and chiefly produce defects in primary hemostasis. The severity of symptoms primarily depends on 2 variables: (1) the identity of the deficient or defective protein, and (2) the extent of the deficiency or functional defect. Severe deficiencies manifest themselves early during childhood, with frequent episodes of mucocutaneous bleeding, such as purpura, gingival bleeding, epistaxis, menorrhagia, and prolonged bleeding after trauma or surgery. Hematuria and gastrointestinal bleeding, and rarely intracranial hemorrhage may occur spontaneously. Mild deficiencies may not be diagnosed until adulthood or until the hemostatic system is stressed by surgery or trauma. Inherited platelet disorders can also be associated with other clinical features, such as skeletal abnormalities and mental retardation in velo-cardiofacial syndrome, hearing loss, or renal disorders. In some patients, the presence of thrombocytopenia with large platelets may lead to the misdiagnosis of immune thrombocytopenia (ITP) and lead to unnecessary treatments. Family history, platelet aggregation tests, flow cytometric analysis of platelet surface glycoproteins, and genetic analysis discriminate inherited disorders from acquired ones, although not always with 100% certainty.
Inherited platelet disorders are a large and heterogeneous group of diseases caused by genetic mutations of a large number genes, some expressed exclusively on platelets and megakaryocytes, and some having more widespread distribution. An overview of inherited platelet function disorders and a review of disorders of platelet granules and secretion is presented elsewhere in this issue. Inherited thrombocytopenias are described in a separate review. This review primarily focuses on inherited defects of platelet membrane glycoproteins.
Disorders of the glycoprotein Ib-IX-V complex
The glycoprotein (GP) Ib-IX-V complex is constitutively expressed on platelets and megakaryocytes and mediates several important platelet interactions with other molecules. The GPIb-IX-V complex contains 4 distinct polypeptide subunits in a stoichiometry of 2 GPIbα, 4 GPIbβ, 2 GPIX, and 1 molecule of GPV. Although this stoichiometry has been established, the exact number of subunits in a functional complex has not been. Each subunit has the structure of a type I transmembrane protein, with a single transmembrane domain separating an extracellular N-terminus from an intracellular C-terminus. Each also belongs to the leucine-rich repeat (LRR) superfamily of proteins that includes, prominently, the toll-like receptors. Whether this shared ancestry implies anything about the functions of the GPIb-IX-V complex is not clear. The LRRs reside in the extracellular portion of the polypeptides, where they are flanked by disulfide loops at the N- and C-termini. In GPIbα, the polypeptide that contains the binding sites for all of the known ligands of the complex, the LRR-containing ligand-binding domain is separated from the platelet plasma membrane by a extended, highly glycosylated mucin core. Each polypeptide also has a cytoplasmic domain through which the polypeptides associate with cytoskeletal elements, such as filamin A, and adapter and signaling proteins, such as 14-3-3ζ, calmodulin, and phosphoinositide-3 kinase. The 4 subunits are encoded by different genes. After transcription and translation of the polypeptides, they associate to produce the receptor complex in the endoplasmic reticulum. Posttranslational modifications of the molecule are very important for the functions of the receptor and include extensive N- and O-glycosylation, palmitoylation of GPIbβ and GPIX, and tyrosine sulfation. Approximately 15,000 to 25,000 copies of GPIb-IX-V complex are expressed on human platelets.
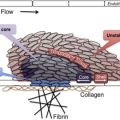
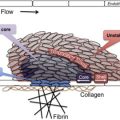
The GPIb-IX-V complex mediates several interactions of importance in thrombosis and hemostasis. GPIbα binds von Willebrand Factor (VWF), thrombin, P-selectin, Mac-1, factor XI, factor XII, high molecular weight kininogen, thrombospondin, and β-2 glycoprotein I. Of these, the interaction with VWF, and possibly thrombin, appear to be the most important, as judged by the phenotype of the deficiency syndrome. Binding of GPIbα to VWF is the key event in the adhesion of platelets to the subendothelium, which is exposed with traumatic vessel injury or rupture of atherosclerotic plaques. Matrix-bound VWF, unlike VWF circulating in blood, expresses a normally cryptic GPIbα binding site, allowing platelets from the blood to adhere and spread at the site of injury, with subsequent aggregation mediated by other membrane glycoproteins. Two other circumstances in addition to being immobilized on the subendothelial surface also allow VWF to bind GPIbα: (1) exposure of plasma VWF to very high shear stresses, where the force unfolds VWF and exposes the GPIbα binding site; and (2) acute release and defective removal of ultralarge VWF multimers from the endothelial surface, as occurs in thrombotic thrombocytopenic purpura. Recent data indicate that the GPIbα–VWF interaction may also be important in the pathogenesis of venous thrombosis. Interactions between GPIbα and coagulation proteins, such as thrombin, factor XI, and factor XII, may also influence the activity of the coagulation cascade on the platelet surface. The GPIb-IX-V complex also has a role in inflammatory reactions, as indicated by its ability to bind both P-selectin and Mac-1, and the anti-inflammatory effects of blocking those interactions in experimental models.
Inherited mutations of the platelet GPIb-IX-V complex either prevent its expression on the platelet surface or create a dysfunctional receptor. Absence of the receptor on the cell surface, or mutations that interfere with VWF binding, produce Bernard-Soulier syndrome. GPIbα can also be affected by mutations that increase the affinity of the interaction with VWF, producing the bleeding disorder platelet-type von Willebrand disease.
Bernard-Soulier Syndrome
This disorder, originally described by the French physicians Jean Bernard and Jean-Pierre Soulier in 1948, is characterized by a bleeding tendency, thrombocytopenia, giant platelets, and the absence of ristocetin-induced platelet agglutination. It is an orphan disease, with an estimated prevalence of fewer than 1 in 1 million.
Patients with BSS generally present with manifestations of mucocutaneous bleeding (purpura, gingival bleeding, epistaxis, menorrhagia) and prolonged and excessive bleeding after trauma or surgery, usually beginning in early childhood. Spontaneous gastrointestinal or urogenital bleeding may occur; intracranial bleeding and intraperitoneal hematomas are very rare. Bleeding signs and symptoms may vary considerably among affected individuals, even within the same family.
Thrombocytopenia and giant platelets on the blood smear are important features of the disease. Platelet counts range from 10 to 200 × 10 9 . The reason that GPIb-IX-V complex deficiency causes thrombocytopenia and large platelets is not understood, but it has been speculated that the large platelets are a consequence of defective attachment of the plasma membrane to the membrane skeleton, an association largely mediated by the GPIb-IX-V complex. Some of the thrombocytopenia is accounted for by the fact that giant platelets may not be recognized by automatic cell counters, which may count them as leukocytes instead. Although some studies showed platelet survival time to be decreased in patients with BSS, the results are inconsistent and some patients have normal platelet half-lives. The difficulty of isolating giant platelets from blood samples may affect the survival studies. Animal models and some studies with patients with BSS have shown that the absence of the GPIb-IX-V complex impairs megakaryocyte membrane development, alters tubulin distribution, and causes abnormal proplatelet formation with production of giant platelets. Thrombocytopenia and defective platelet adhesion together account for the hemostatic defect. Prolonged closure times using the platelet function analyzer (PFA)-100, which measures shear-dependent platelet functions in vitro using both collagen/ADP and collagen/epinephrine embedded cartridges, is sensitive in detecting the hemostatic defect of BSS.
Iron deficiency anemia may occur in patients with BSS who experience severe bleeding, especially women with menorrhagia. The erythrocytes are otherwise normal. Leukocyte counts and morphology should be carefully evaluated in patients with giant platelets for discrimination of MYH9-related diseases. Coagulation tests are also normal, including clot retraction, prothrombin time, activated partial thromboplastin time, and levels of VWF and factor VIII. Although they are not routinely tested, prothrombin consumption and thrombin generation are decreased in BSS. The defect in thrombin consumption can be mimicked by treating normal platelets with a GPIbα antibody that blocks VWF binding.
Unfortunately, routine tests cannot differentiate BSS from other inherited and acquired giant platelet syndromes. BSS is most often misdiagnosed as immune thrombocytopenia (ITP), because macrothrombocytopenia is often found in patients with ITP. This is especially true in carriers of BSS mutations. A family history of bleeding and personal history of bleeding signs and symptoms since early childhood weigh against a diagnosis of ITP. Platelet aggregation tests and platelet surface glycoprotein analysis are crucial for diagnosis of BSS. In aggregation studies, BSS platelets characteristically show lack of ristocetin-induced agglutination, but have normal or near-normal responses to collagen, ADP, and epinephrine. Aggregation is defective at low concentrations of thrombin, but normal at higher concentrations. Flow cytometric analysis of platelet surface glycoproteins by immunostaining usually reveals severe deficiency or absence of the GPIb-IX-V complex. Other specific tests, such as immunoblotting after sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis may show absence of specific fragments of the receptor. In a special subtype, the Bolzano variant, GPIb-IX-V complex is present on the platelet surface in normal quantities, but the receptor cannot bind VWF because of mutation of Ala 156 to Val in GPIbα.
BSS is almost always inherited as an autosomal recessive trait, and often associated with consanguinity. Autosomal dominant inheritance has also been reported, but much less frequently. Mutations responsible for BSS are usually specific to a particular patient or family. Heterozygous individuals have reduced GPIb-IX-V complex on the platelet surface, and usually do not have bleeding symptoms, but may manifest macrothrombocytopenia. In Italy, heterozygosity for BSS is the most common cause of inherited macrothrombocytopenia.
Mutations responsible for BSS are highly heterogeneous and include nonsense mutations, missense mutations, and frameshift insertions or deletions involving the genes encoding GPIbα, GPIbβ, or GPIX. Mutations of the GPV gene have not been described in BSS, and its gene product is unnecessary for expression of a functional complex in transfected cells.
Platelet-type von Willebrand disease
Platelet-type von Willebrand disease (VWD) (also called pseudo-VWD) is caused by dominantly inherited gain-of-function mutations in GPIbα. Four point mutations (G233V, G233S, D235Y, M239V) and a 27–base pair deletion have been described in patients with platelet-type VWD. These mutations increase the affinity of GPIbα for VWF, allowing VWF to bind spontaneously to platelets (no modulator required), resulting in clearance of the highest-molecular-weight and hemostatically most active multimers of VWF from the plasma and producing a bleeding diathesis. Bleeding symptoms are usually mild to moderate but can become life threatening after surgery, during pregnancy, or with the use of antiplatelet drugs. The blood smear usually displays mild thrombocytopenia and large platelets. Platelet-type VWD is often misdiagnosed as ITP or type 2B VWD. The presence of increased ristocetin-induced platelet aggregation (RIPA), decreased ristocetin cofactor activity of the plasma, and normal or mildly decreased VWF antigen levels help to exclude ITP. On the other hand, discrimination of platelet-type VWD from type 2B VWD can be complicated. The RIPA mixing assay, flow cytometry, and genetic analysis of VWF (for type 2B VWD) and GPIbα (for platelet-type VWD) are used for the exact diagnosis. In the RIPA mixing assay, combining patient platelets with normal plasma yields RIPA results similar to those with patient platelet-rich plasma. Management of these 2 diseases is also different: bleeding is controlled by platelet transfusions in patients with platelet-type VWD, whereas VWF-containing preparations are used in patients with type 2B VWD.
Velo-cardiofacial syndrome
BSS-like functional defects may be seen in patients with velo-cardiofacial syndrome (VCFS). VCFS is a developmental disorder characterized by abnormal development of the pharyngeal arch. Multiple abnormalities, such as craniofacial defects, cardiac abnormalities, immune deficiencies, and mental problems are present in different variants. VCFS is caused by deletions in chromosome 22q11. Because this region contains the gene encoding GPIbβ, patients with VCFS may have increased platelet size, thrombocytopenia, and reduced aggregation with ristocetin.
GPIbα polymorphisms
Several polymorphisms of GPIbα have been described. Because GPIbα is the receptor that mediates platelet adhesion, these polymorphisms have been studied extensively to examine their link with arterial thrombosis.
The GPIbα variable number of tandem repeat polymorphism
The GPIbα variable number of tandem repeat (VNTR) polymorphism affects the region encoding the macroglycopeptide of GPIbα, which contains a variable number of 39–base pair tandem repeats, each encoding identical 13–amino acid sequences. Four polymorphic variants have been identified. In order of decreasing size these are: A, B, C, and D, with 4, 3, 2, and 1 repeat, respectively. The number of repeats affects the length of the macroglycopeptide and the degree to which the ligand-binding domain protrudes above the plasma membrane, and thereby may change the ability of GPIbα to bind VWF. The GPIbα VNTR polymorphism has been extensively studied in patients with arterial thrombosis, with inconsistent results.
HPA-2 (Ko a/b ) polymorphism of GPIbα
Human platelet allo-antigen HPA-2, also known as the Ko polymorphism, is a threonine/methionine dimorphism at position 145 of GPIbα. Threonine is the most prevalent amino acid at this position in all populations tested. The HPA-2 polymorphism is associated with platelet transfusion refractoriness and neonatal alloimmune thrombocytopenia. The polymorphic site is located near the VWF and thrombin binding sites of GPIbα. The HPA-2 polymorphism is in linkage disequilibrium with the GPIbα VNTR polymorphism.
Kozak sequence polymorphism of the GPIBA gene
The Kozak sequence polymorphism of the GPIBA gene is based on the presence of either thymine or cytosine at position –5 from the initiator ATG codon and therefore does not change the amino acid sequence of the polypeptide. The frequency of the less prevalent C allele ranges from 8% to 17% in different ethnic groups. The presence of the C allele correlated with increased mRNA translation and higher surface expression of GPIbα in affected individuals. The impact of the Kozak polymorphism has been investigated in patients with arterial thrombosis with different results. Some investigators showed an association of the C allele with coronary thrombosis and stroke ; others found no association. An increased risk of thrombosis linked to the Kozak polymorphism was reported in postmenopausal women taking hormone replacement therapy and in patients with antiphospholipid syndrome.
Disorders of the glycoprotein Ib-IX-V complex
The glycoprotein (GP) Ib-IX-V complex is constitutively expressed on platelets and megakaryocytes and mediates several important platelet interactions with other molecules. The GPIb-IX-V complex contains 4 distinct polypeptide subunits in a stoichiometry of 2 GPIbα, 4 GPIbβ, 2 GPIX, and 1 molecule of GPV. Although this stoichiometry has been established, the exact number of subunits in a functional complex has not been. Each subunit has the structure of a type I transmembrane protein, with a single transmembrane domain separating an extracellular N-terminus from an intracellular C-terminus. Each also belongs to the leucine-rich repeat (LRR) superfamily of proteins that includes, prominently, the toll-like receptors. Whether this shared ancestry implies anything about the functions of the GPIb-IX-V complex is not clear. The LRRs reside in the extracellular portion of the polypeptides, where they are flanked by disulfide loops at the N- and C-termini. In GPIbα, the polypeptide that contains the binding sites for all of the known ligands of the complex, the LRR-containing ligand-binding domain is separated from the platelet plasma membrane by a extended, highly glycosylated mucin core. Each polypeptide also has a cytoplasmic domain through which the polypeptides associate with cytoskeletal elements, such as filamin A, and adapter and signaling proteins, such as 14-3-3ζ, calmodulin, and phosphoinositide-3 kinase. The 4 subunits are encoded by different genes. After transcription and translation of the polypeptides, they associate to produce the receptor complex in the endoplasmic reticulum. Posttranslational modifications of the molecule are very important for the functions of the receptor and include extensive N- and O-glycosylation, palmitoylation of GPIbβ and GPIX, and tyrosine sulfation. Approximately 15,000 to 25,000 copies of GPIb-IX-V complex are expressed on human platelets.
The GPIb-IX-V complex mediates several interactions of importance in thrombosis and hemostasis. GPIbα binds von Willebrand Factor (VWF), thrombin, P-selectin, Mac-1, factor XI, factor XII, high molecular weight kininogen, thrombospondin, and β-2 glycoprotein I. Of these, the interaction with VWF, and possibly thrombin, appear to be the most important, as judged by the phenotype of the deficiency syndrome. Binding of GPIbα to VWF is the key event in the adhesion of platelets to the subendothelium, which is exposed with traumatic vessel injury or rupture of atherosclerotic plaques. Matrix-bound VWF, unlike VWF circulating in blood, expresses a normally cryptic GPIbα binding site, allowing platelets from the blood to adhere and spread at the site of injury, with subsequent aggregation mediated by other membrane glycoproteins. Two other circumstances in addition to being immobilized on the subendothelial surface also allow VWF to bind GPIbα: (1) exposure of plasma VWF to very high shear stresses, where the force unfolds VWF and exposes the GPIbα binding site; and (2) acute release and defective removal of ultralarge VWF multimers from the endothelial surface, as occurs in thrombotic thrombocytopenic purpura. Recent data indicate that the GPIbα–VWF interaction may also be important in the pathogenesis of venous thrombosis. Interactions between GPIbα and coagulation proteins, such as thrombin, factor XI, and factor XII, may also influence the activity of the coagulation cascade on the platelet surface. The GPIb-IX-V complex also has a role in inflammatory reactions, as indicated by its ability to bind both P-selectin and Mac-1, and the anti-inflammatory effects of blocking those interactions in experimental models.
Inherited mutations of the platelet GPIb-IX-V complex either prevent its expression on the platelet surface or create a dysfunctional receptor. Absence of the receptor on the cell surface, or mutations that interfere with VWF binding, produce Bernard-Soulier syndrome. GPIbα can also be affected by mutations that increase the affinity of the interaction with VWF, producing the bleeding disorder platelet-type von Willebrand disease.
Bernard-Soulier Syndrome
This disorder, originally described by the French physicians Jean Bernard and Jean-Pierre Soulier in 1948, is characterized by a bleeding tendency, thrombocytopenia, giant platelets, and the absence of ristocetin-induced platelet agglutination. It is an orphan disease, with an estimated prevalence of fewer than 1 in 1 million.
Patients with BSS generally present with manifestations of mucocutaneous bleeding (purpura, gingival bleeding, epistaxis, menorrhagia) and prolonged and excessive bleeding after trauma or surgery, usually beginning in early childhood. Spontaneous gastrointestinal or urogenital bleeding may occur; intracranial bleeding and intraperitoneal hematomas are very rare. Bleeding signs and symptoms may vary considerably among affected individuals, even within the same family.
Thrombocytopenia and giant platelets on the blood smear are important features of the disease. Platelet counts range from 10 to 200 × 10 9 . The reason that GPIb-IX-V complex deficiency causes thrombocytopenia and large platelets is not understood, but it has been speculated that the large platelets are a consequence of defective attachment of the plasma membrane to the membrane skeleton, an association largely mediated by the GPIb-IX-V complex. Some of the thrombocytopenia is accounted for by the fact that giant platelets may not be recognized by automatic cell counters, which may count them as leukocytes instead. Although some studies showed platelet survival time to be decreased in patients with BSS, the results are inconsistent and some patients have normal platelet half-lives. The difficulty of isolating giant platelets from blood samples may affect the survival studies. Animal models and some studies with patients with BSS have shown that the absence of the GPIb-IX-V complex impairs megakaryocyte membrane development, alters tubulin distribution, and causes abnormal proplatelet formation with production of giant platelets. Thrombocytopenia and defective platelet adhesion together account for the hemostatic defect. Prolonged closure times using the platelet function analyzer (PFA)-100, which measures shear-dependent platelet functions in vitro using both collagen/ADP and collagen/epinephrine embedded cartridges, is sensitive in detecting the hemostatic defect of BSS.
Iron deficiency anemia may occur in patients with BSS who experience severe bleeding, especially women with menorrhagia. The erythrocytes are otherwise normal. Leukocyte counts and morphology should be carefully evaluated in patients with giant platelets for discrimination of MYH9-related diseases. Coagulation tests are also normal, including clot retraction, prothrombin time, activated partial thromboplastin time, and levels of VWF and factor VIII. Although they are not routinely tested, prothrombin consumption and thrombin generation are decreased in BSS. The defect in thrombin consumption can be mimicked by treating normal platelets with a GPIbα antibody that blocks VWF binding.
Unfortunately, routine tests cannot differentiate BSS from other inherited and acquired giant platelet syndromes. BSS is most often misdiagnosed as immune thrombocytopenia (ITP), because macrothrombocytopenia is often found in patients with ITP. This is especially true in carriers of BSS mutations. A family history of bleeding and personal history of bleeding signs and symptoms since early childhood weigh against a diagnosis of ITP. Platelet aggregation tests and platelet surface glycoprotein analysis are crucial for diagnosis of BSS. In aggregation studies, BSS platelets characteristically show lack of ristocetin-induced agglutination, but have normal or near-normal responses to collagen, ADP, and epinephrine. Aggregation is defective at low concentrations of thrombin, but normal at higher concentrations. Flow cytometric analysis of platelet surface glycoproteins by immunostaining usually reveals severe deficiency or absence of the GPIb-IX-V complex. Other specific tests, such as immunoblotting after sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis may show absence of specific fragments of the receptor. In a special subtype, the Bolzano variant, GPIb-IX-V complex is present on the platelet surface in normal quantities, but the receptor cannot bind VWF because of mutation of Ala 156 to Val in GPIbα.
BSS is almost always inherited as an autosomal recessive trait, and often associated with consanguinity. Autosomal dominant inheritance has also been reported, but much less frequently. Mutations responsible for BSS are usually specific to a particular patient or family. Heterozygous individuals have reduced GPIb-IX-V complex on the platelet surface, and usually do not have bleeding symptoms, but may manifest macrothrombocytopenia. In Italy, heterozygosity for BSS is the most common cause of inherited macrothrombocytopenia.
Mutations responsible for BSS are highly heterogeneous and include nonsense mutations, missense mutations, and frameshift insertions or deletions involving the genes encoding GPIbα, GPIbβ, or GPIX. Mutations of the GPV gene have not been described in BSS, and its gene product is unnecessary for expression of a functional complex in transfected cells.
Platelet-type von Willebrand disease
Platelet-type von Willebrand disease (VWD) (also called pseudo-VWD) is caused by dominantly inherited gain-of-function mutations in GPIbα. Four point mutations (G233V, G233S, D235Y, M239V) and a 27–base pair deletion have been described in patients with platelet-type VWD. These mutations increase the affinity of GPIbα for VWF, allowing VWF to bind spontaneously to platelets (no modulator required), resulting in clearance of the highest-molecular-weight and hemostatically most active multimers of VWF from the plasma and producing a bleeding diathesis. Bleeding symptoms are usually mild to moderate but can become life threatening after surgery, during pregnancy, or with the use of antiplatelet drugs. The blood smear usually displays mild thrombocytopenia and large platelets. Platelet-type VWD is often misdiagnosed as ITP or type 2B VWD. The presence of increased ristocetin-induced platelet aggregation (RIPA), decreased ristocetin cofactor activity of the plasma, and normal or mildly decreased VWF antigen levels help to exclude ITP. On the other hand, discrimination of platelet-type VWD from type 2B VWD can be complicated. The RIPA mixing assay, flow cytometry, and genetic analysis of VWF (for type 2B VWD) and GPIbα (for platelet-type VWD) are used for the exact diagnosis. In the RIPA mixing assay, combining patient platelets with normal plasma yields RIPA results similar to those with patient platelet-rich plasma. Management of these 2 diseases is also different: bleeding is controlled by platelet transfusions in patients with platelet-type VWD, whereas VWF-containing preparations are used in patients with type 2B VWD.
Velo-cardiofacial syndrome
BSS-like functional defects may be seen in patients with velo-cardiofacial syndrome (VCFS). VCFS is a developmental disorder characterized by abnormal development of the pharyngeal arch. Multiple abnormalities, such as craniofacial defects, cardiac abnormalities, immune deficiencies, and mental problems are present in different variants. VCFS is caused by deletions in chromosome 22q11. Because this region contains the gene encoding GPIbβ, patients with VCFS may have increased platelet size, thrombocytopenia, and reduced aggregation with ristocetin.
GPIbα polymorphisms
Several polymorphisms of GPIbα have been described. Because GPIbα is the receptor that mediates platelet adhesion, these polymorphisms have been studied extensively to examine their link with arterial thrombosis.
The GPIbα variable number of tandem repeat polymorphism
The GPIbα variable number of tandem repeat (VNTR) polymorphism affects the region encoding the macroglycopeptide of GPIbα, which contains a variable number of 39–base pair tandem repeats, each encoding identical 13–amino acid sequences. Four polymorphic variants have been identified. In order of decreasing size these are: A, B, C, and D, with 4, 3, 2, and 1 repeat, respectively. The number of repeats affects the length of the macroglycopeptide and the degree to which the ligand-binding domain protrudes above the plasma membrane, and thereby may change the ability of GPIbα to bind VWF. The GPIbα VNTR polymorphism has been extensively studied in patients with arterial thrombosis, with inconsistent results.
HPA-2 (Ko a/b ) polymorphism of GPIbα
Human platelet allo-antigen HPA-2, also known as the Ko polymorphism, is a threonine/methionine dimorphism at position 145 of GPIbα. Threonine is the most prevalent amino acid at this position in all populations tested. The HPA-2 polymorphism is associated with platelet transfusion refractoriness and neonatal alloimmune thrombocytopenia. The polymorphic site is located near the VWF and thrombin binding sites of GPIbα. The HPA-2 polymorphism is in linkage disequilibrium with the GPIbα VNTR polymorphism.
Kozak sequence polymorphism of the GPIBA gene
The Kozak sequence polymorphism of the GPIBA gene is based on the presence of either thymine or cytosine at position –5 from the initiator ATG codon and therefore does not change the amino acid sequence of the polypeptide. The frequency of the less prevalent C allele ranges from 8% to 17% in different ethnic groups. The presence of the C allele correlated with increased mRNA translation and higher surface expression of GPIbα in affected individuals. The impact of the Kozak polymorphism has been investigated in patients with arterial thrombosis with different results. Some investigators showed an association of the C allele with coronary thrombosis and stroke ; others found no association. An increased risk of thrombosis linked to the Kozak polymorphism was reported in postmenopausal women taking hormone replacement therapy and in patients with antiphospholipid syndrome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree