Fig. 15.1
(a) Punch biopsy of the skin demonstrates small-, medium-, and large-sized lymphovascular tumor emboli and the superficial dermis from a patient with clinical manifestation of IBC. (b) The tumor cells forming the emboli demonstrate high nuclear grade. Note the presence of a mild lymphoplamacytic infiltration in between the emboli
Epidemiology and Risk Factors
A large population-based study describing the demographic and tumor characteristics of 3,626 women with IBC diagnosis during 1994–1998 demonstrated that the majority of the cases were between 40 and 59 years old [15]. This study also revealed that the incidence of IBC was 1.3 per 100,000 for all races combined. African American women had the highest relative risk (1.6) and Asian and Pacific Islanders women had the lowest (0.7). A large analysis from SEER population-based cancer registry described a marked epidemiologic difference between IBC and LABC [2]. The presence of young age at diagnosis, high nuclear grade, and absence of estrogen receptor (ER)-positive are suggestive of IBC rather than LABC. In a multivariate analysis after adjusting for race, age, tumor size, axillary lymph node status, histologic grade, and ER expression, the risk of death from IBC was nearly twice that compared to LABC [2]. A recent study analyzing the California Cancer Registry of 2,014 IBC patients found that despite an association with stage, HER2-positive status was not an independent adverse prognostic factor for survival among IBC patient cases [16]. These differences in prognostic factor profiles and age-specific incidence patterns support the hypothesis that IBC and LABC are distinct biologic entities.
The number of cases of IBC in Western countries is low; however, they are increasing in overall incidence. There appears to be a striking geographic pattern, with a higher incidence in North African countries (Maghreb) especially Morocco, Algeria, Tunisia, and Egypt, with the incidence of IBC reported to be 10–15 % [17]. However, in these countries, there is some uncertainty about the completeness of case registration and strict definitions used for IBC detection. There are very limited published data on the risk factors for developing IBC. A report from SEER that contains the largest population-based sample of IBC in the United States was published in 1999 [18]. During the period 1975–1981, IBC patients were younger at diagnosis than non-IBC patients. African American women tended to be younger than white women, and the 3-year survival rate for patients with IBC was far lower (34 %) than that for patients with other types of carcinoma (90 %). A second report from SEER demonstrated that between 1975–1977 and 1990–1992, the overall age-adjusted incidence of IBC doubled, increasing among white women from 0.3 to 0.7 cases per 100,000 person-years and among African Americans from 0.6 to 1.1 [1]. Patients with IBC are affected at an early age. Among white women, the mean age at diagnosis for IBC patients (mean = 57 years) was significantly younger than that for other breast carcinoma (62 years, p = 0.0001). Among African Americans, IBC were significantly younger (mean = 52 years) than other breast carcinoma patients (mean = 57 years, p = 0.0003) [1].
High body mass index (BMI) has been associated with a lower risk of premenopausal breast cancer [19, 20], however, a higher risk of postmenopausal breast cancer [21]. This observation suggests that reproductive hormones and factors related to the body size may partially contribute to the overall risk of developing breast cancer. A single institution report evaluated the BMI as a risk factor for IBC. In this study, a comparison of 68 IBC patients, 143 non-IBC cases, and 134 non-breast cancer cases suggested that IBC patients have an earlier age at menarche and a greater BMI [1]. IBC patients were younger at menarche and at the time of their first live birth than non-IBC and non-breast cancer patients.
The proportion of premenopausal IBC patients was higher than the proportion of premenopausal women in the comparison groups, although differences were not statistically significant. There were no differences in height, but IBC patients were heavier (77.6 kg) than non-IBC (70.0 kg) and non-breast cancer patients (68.0 kg). After adjusting for other factors, women in the highest BMI percentile (BMI >26.65 kg/m2) relative to the lowest percentile (BMI <22.27) had significantly increased IBC risk (IBC vs. non-IBC, odds ratio [OR] = 2.45, 95 % CI, 1.05–5.73; IBC vs. non-breast cancer, OR = 4.52, 95 % CI = 1.85–11.04). This association was not significantly modified by menopausal status and was independent of age at menarche, family history of breast cancer, gravidity, smoking status, and alcohol use.
In 1936, Bittner and collaborators [22] described the involvement of the mouse mammary tumor virus (MMTV) in mouse mammary carcinogenesis. Subsequently, the sequences of MMTV-like were described in breast cancer samples but absent in normal tissues in multiple reports. More recently, Pogo and collaborators [23] found that retroviral sequences of the MMTV were present in 40 % of the sporadic breast cancer contrasting with 71 % of IBC in American women. Similar incidence has been found in IBC cancers from Tunisia. Because these conditions represent highly invasive malignancies, it is concluded that HMTV is sometimes associated with a particularly malignant phenotype.
Molecular Insights of IBC
The classical description of IBC attributes to the obstruction of lymphatic vessels the clinical aspect of inflammation. However, the mechanism of “inflammatory” symptoms may be related to a local release of multiple tumor-derived inflammatory cytokines. Besides established and key angiogenic factors like VEGF, chemokines, a superfamily of cytokine-like proteins that bind to seven transmembrane-spanning G protein-coupled receptors, have been associated with angiogenesis under homeostatic conditions. Chemokine receptors, CXCR4 and CCR7, are highly expressed in human breast cancer cells, and metastases. Their respective ligands CXCL12/SDF-1α and CCL21/6Ckine exhibit peak levels of expression in organs representing the first destination of breast cancer metastasis [24].
IBC is characterized by the expression of multiple chemokine receptors, with Cabliogu et al. [24] reporting on IBC patients found to exhibit high levels of expression of CXCR4, EFGR, and HER2-neu amplification. Increased expression of cytoplasmic CXCR4 in almost 50 % of the IBC samples compared with only 5 % expression of T1-tumors lymph node negative, and 11 % of T1-tumors lymph node positive, appears to be at least partially responsible for the metastatic process [25]. EGFR overexpression was detected in 30 % of IBC patients by immunohistochemical staining [24], and it was associated with a higher increase of recurrence and significantly worse 5-year overall survival rate compared to EGFR-negative IBC. Interestingly, co-expression of CXCR4 and growth factor receptors, particularly HER2-neu and EGFR in breast cancer, has been associated with poor outcome. Increased expression of HER2-neu and EGFR in IBC compared with non-IBC appears to be more specific to the IBC phenotype [25].
Experimental models have recently led to the identification of genes involved in IBC, such as ARHC, coding for the RhoC GTPase, and WISP3, coding for S-glutathione-related protein [26]. In human studies, comparing patients with IBC with stage-matched, non-IBC tumor samples identifies two important genetic characteristics of IBC: loss of WISP3 and overexpression of RhoGTPase [27]. WISP3 is a tumor suppressor gene that produces proteins that are biologically important, such as with cell proliferation, migration, wound healing, angiogenesis, and carcinogenesis [28]. Expression of WISP3 was lost in 80 % of the IBC samples versus only 20 % of the stage-matched, non-IBC tumors [29]. In preclinical models, using the IBC cell line SUM149, restoring WISP3 gene expression decreased tumor cell growth, invasiveness, and angiogenic potential [30]. RhoC GTPase is a member of the Ras superfamily of small GTP-binding proteins and contributes to the metastatic characteristics of IBC by upregulation of angiogenic factors (VEGF and bFGF) promoting cell motility and invasion [31, 32]. In a comparative study of human tumor samples with stage-matched, non-IBC samples, RhoC was overexpressed in 90 % of the IBC tumors versus 38 % of the non-IBC tumors [27].
Cadherins are integral membrane glycoproteins that mediate calcium-dependent cell-cell adhesion and being responsible of the intercellular signaling trafficking. Loss of E-cadherin expression has been related to a wide spectrum of human cancers, especially prostate and breast. Alterations in the cadherin complexes are directly implicated in tumorigenesis and cancer progression [33]. E-cadherin was found overexpressed in IBC, and strong expression was observed in lymphovascular tumor emboli from IBC [34]. Preclinical data using antibodies against E-cadherin caused dissolution of pulmonary lymphovascular emboli in an IBC xenograft model [35]. The biological role of E-cadherin is not completely known. On the basis of these results, we could hypothesize that the loss of E-cadherin occurs in the early phase of IBC as a transient effect to induce epithelial-to-mesenchymal transition and allow metastasis and that, by the time of diagnosis of IBC, tumor cells have reinstated expression of E-cadherin [29].
It has been validated through many studies that in vitro and in vivo tumor models of IBC have a high expression of proangiogenic and pro-lymphangiogenic molecules. One of the pioneering studies showed an increased mRNA expression of VEGF-C, VEGF-D, KDR, Flt-4, Ang-1, Tie-1, Tie-2, cyclooxygenease-2, fibroblast growth factor-2 (FGF-2), Prox-2, and LYVE-1 in 16 IBC compared with 20 non-IBC specimens. These factors support the rapid growth of tumor cells under hypoxic conditions and also promote a venue for dissemination. In addition to the classic angiogenic pathways associated with endothelial migration, proliferation, and organization to form new vessels, driven primarily by VEGF and its receptors, IBC tumors exhibit vasculogenesis, which is the de novo formation of vessel-like structures that allow the flow of oxygen and nutrients in the absence of endothelial cells.
The ability of tumor cells to form tubelike structures is defined as vasculogenic mimicry. Evaluation of molecules and pathways that are known to regulate vasculogenesis and lymphangiogenesis represents a very exciting area of drug development and the clinical studies with these new molecules providing new opportunities for understanding the spectrum of angiogenesis that is crucial to the distinct molecular signature of IBC. The anaplastic lymphoma kinase (ALK) gene belongs to the insulin receptor superfamily and encodes a receptor tyrosine kinase that is normally expressed only in select neuronal cell types [36]. Aberrant ALK activity results from point mutations, amplifications, chromosomal translocations, or other types of gene rearrangements involving the ALK gene.
Mutations in the ALK gene have been associated with several cancers and chromosomal translocations linking ALK to their fusion partners in anaplastic large cell lymphoma (ALCL), inflammatory myofibroblastic tumors, and neuroblastoma [37]. Recently, a novel gene fusion involving ALK and echinoderm microtubule-associated protein-like 4 (EML4) was discovered in non-small cell lung cancer (NSCLC), and treatment with ALK inhibitors in vitro has been reported to lead to cell cycle arrest and apoptosis. A recent preliminary report revealed evidence for amplification (three- to sevenfold) of the ALK receptor in 13/15 IBC patients and 66 % amplification by FISH in IBC cell lines [38]. A phase I, multicenter study of LDK378 in patients with genetic abnormalities in ALK is currently ongoing (NCT01283516).
Histone deacetylase (HDACs) cooperate with histone acetyltransferases (HATs) to regulate the acetylation status of nuclear histones, transcription factors, and other cellular proteins to regulate a variety of cellular processes including cell division and gene expression and cell death [39]. HDAC inhibitors are also currently being evaluated for their therapeutic potential in breast cancer.
Clinical Characteristics
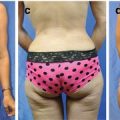
Patients with IBC typically present with a sudden onset of increase in size of the breast, firmness, tenderness, and redness of the skin overlying the breast. In a seminal publication by Haagensen et al. [13], they described the clinical presentation and associated symptoms of IBC including a breast mass (57 %), redness of the skin (57 %), breast enlargement (48 %), pain in the breast or nipple (29 %), breast tenderness (16 %), generalized breast hardness (16 %), nipple retraction (13 %), edema of the skin (13 %), axillary mass (9 %), and warmness of the skin (8 %). In this analysis, the median duration of these symptoms before the diagnosis of IBC was 2.5 months, compared with 5 months for non-IBCs (Fig. 15.2a, b). Several breast diseases may mimic IBC and this might result in a delay of diagnosis. The two most common are infectious mastitis and breast abscess, both of which can be associated with lactation, skin erythema and redness, fever, and leukocytosis. Ductal ectasia can also mimic IBC, characterized by localized inflammation and enlarged breast that responds quickly to supportive measures.

Fig. 15.2
There are variations in clinical presentation of IBC. Patient (a) presented with synchronous bilateral erythema. Patient (b) is an IBC patient with increased breast size, peau d’orange, and minimal erythema on background of darker skin
Imaging Studies
Evaluation of the patient with IBC involves a diagnostic mammogram, which is almost always abnormal [40]. The most common mammographic characteristics of IBC consist in skin thickening and diffusely increased density in 92 and 81 % of patients, respectively [41]. The authors defined a focal asymmetric density as asymmetry of tissue density, but completely lacking borders and the conspicuity of a true mass. In the same series, axillary lymphadenopathy was seen in 58 % of patients. A series including 22 patients with IBC from Memorial Sloan-Kettering Cancer Center found that 95 % of patients had a breast mass or malignant-appearing calcifications identified on mammography [42]. In a comparative study of PET/CT, magnetic resonance imaging (MRI), mammography, and sonography of 80 patients with IBC, mammography resulted in the least sensitive imaging method for diagnosing multifocal and multicentric disease [43].
Ultrasonography can be very helpful in the evaluation of regional nodal status. The most common findings with sonography are skin thickening, increased vascularity in the axillary lymphadenopathies, and architectural distortion. In a recent study from MD Anderson Cancer Center, regional axillary nodal disease was diagnosed in 93 % of IBC patients with this modality [43]. This percentage of axillary nodal involvement is highest compared with previous reported series where axillary adenopathy was found in 22–56 % (mean 28 %) [2, 11, 18].
The role of MRI in IBC is currently being investigated, with Chow et al. [44] reporting that the affected breast with IBC was most frequently found to have an infiltrative mass represented by a “reticular/dendritic pattern” of enhancement. Yang et al. [43] also reported that MRI revealed a primary breast lesion in 100 % of the cases, compared with 96 % with combined positron emission tomography and computed tomography (PET/CT), 95 % with ultrasonography, and 80 % with mammography. In this study, the most frequent MRI findings were multiple masses with irregular margins and heterogeneous internal enhancement associated with a washout or plateau kinetic curve in 97 % of patients.
PET/CT is an emerging imaging method that is widely gaining clinical acceptance because of its ability to co-register both anatomic and functional information on one image [45]. PET/CT has the advantage of identifying the local extent of metabolically active carcinomas, as well as lymph node and distant metastases, all in one procedure. A single PET study of seven patients with IBC demonstrated diffusely increased or intense foci of increased uptake in enlarged breast with increased skin uptake [46]. A recent retrospective review from MD Anderson Cancer Center in patients with IBC suggest that MRI would be the preferred initial imaging modality for IBC, and PET/CT would be an excellent companion for the detection of distant metastasis [43].
Multimodality Treatment
More than 70 % of patients with IBC have clinically localized disease without distant metastasis at their initial presentation [15]. A potentially curative, combined modality treatment approach should be offered to this group of patients, demonstrating a very good rate of locoregional control (84 %), with a lower distant metastasis-free survival rate of 47 % and an overall 5-year survival rate of 51 % [47]. In this study of 192 patients treated for IBC at the University of Texas MD Anderson Cancer Center with trimodality therapy including neoadjuvant chemotherapy, mastectomy, and postmastectomy radiation, most often delivered in a dose-dense twice-daily fraction to 66 Gy, revealed a 5-year actuarial locoregional control, distant metastasis-free survival, and overall survival of 84, 47, and 51 %, respectively.
The variable with the strongest relationship with locoregional control was the response to neoadjuvant chemotherapy. The 5-year locoregional control rate was 95 % for the 42 patients with a complete response, 86 % in the 111 patients experiencing a partial response, and 51 % in the 30 patients with less than a partial response (p = 0.0001). Univariate factors significantly associated with locoregional control were response to neoadjuvant chemotherapy, surgical margin status, number of involved lymph nodes, and the use of taxanes. Increasing the total chest wall dose of postmastectomy radiation from 60 to 66 Gy significantly improved locoregional control for patients who experienced less than a partial response to chemotherapy; patients with positive, close, or unknown margins; and patients less than 45 years of age.
Surgical Management
The poor outcome of surgery as the only therapeutic modality was recognized in the 1920s, and observed again in 1950s, resulting in radiotherapy becoming the primary therapeutic treatment modality for the management of IBC until the 1970s [10, 48]. In the 1950s, Hagensen and collaborators [49] reported on 29 patients with IBC treated with radical mastectomy. The mean survival was only 19 months, and no patients were alive at 5 years. Subsequent studies demonstrated that local recurrence, in spite of the combination of chemotherapy, surgery, and radiation, occurred in 25–40 % of the cases.
Modified radical mastectomy has replaced the radical mastectomy as the operative treatment of choice for patients with IBC. However, breast conservation therapy has been used in highly selected patients who achieve a favorable response to neoadjuvant chemotherapy. If residual disease is present within the breast, high radiation dosage (>70 Gy) is required to achieve disease control which may cause substantial tissue damage. Chevalier and collaborators [50] reported a 61 % local failure rate in patients who achieved a complete response to PST and were treated with conservative treatment. Arthur et al. [51] reported slightly higher local control rates among 15 patients who achieved a clinical complete response who were not treated with surgery (87 % at 3 years). However, the follow-up was only 24 months.
Systemic Treatment
Primary Systemic Chemotherapy
The last four decades has seen an evolution in the prognostic outcome of IBC, transforming it from a disease that was once considered uniformly fatal with fewer than 5 % of women surviving past 5 years to rates approaching 40 % [52]. The key component to this transformation was the recognition of the vital importance of a combined modality approach to management, involving from the onset all disciplines of breast cancer management including medical, surgical, and radiation oncology. The recognition of the importance of starting systemic treatment upfront stemmed from the important clinical observation that IBC was, for the most part, not optimally resectable at initial presentation [10]. Furthermore, the disease was often poorly controlled with locoregional modalities alone [53]. The introduction of primary systemic chemotherapy incorporated several advantages: downstaging of the tumor, operability after chemotherapy, in vivo assessment of response to chemotherapy, earlier treatment of subclinical distant micrometastases, and the possibility of obtaining a pathologic complete response. The latter has been shown to be associated with a superior prognostic outcome [54].
Due to the fact that IBC is a rare disease coupled with the fact that it is actively excluded from large clinical trials due to its known associated poor prognostic outcome, most of the available data on systemic management are derived from retrospective studies and small clinical trials. One of the largest series comes from the University of Texas MD Anderson Cancer Center [52] reported on the 20-year experience of a cohort of 178 women with IBC treated on four prospective clinical trials receiving a doxorubicin-based preoperative chemotherapy-based regimen followed by radiation therapy with or without mastectomy. The authors reported 5- and 10-year survival rates of 40 and 33 %, respectively, with 28 % of patients reported to be alive beyond 15 years.
Furthermore, the authors reported 15-year survival rates of 44, 31, and 7 % among patients achieving a clinical complete response, partial response, and less than partial response, respectively, demonstrating the prognostic importance of response to primary systemic therapy. In a more recent review, Baldini et al. [55] reported on a smaller cohort of 68 women with IBC treated with an anthracycline-based regimen (CAF or FEC) followed by surgery, adjuvant chemotherapy, and radiotherapy. Similar to the series from the MD Anderson Cancer Center, the authors reported 5- and 10-year survival rates of 44 and 32 %, respectively. Based on these results and others, an anthracycline-based preoperative chemotherapeutic-based regimen should be considered the current standard of care, as the survival results reported are indeed far superior to those reported historically.
The efficacy of taxanes incorporated into the preoperative anthracycline regimen of patients with IBC has also been explored. In a retrospective study, Cristofanilli and colleagues [56] reported on the results of a comparison of women with IBC who had either been treated with preoperative FAC to those who were treated with preoperative FAC followed by paclitaxel, receiving it either every 3 weeks or weekly. The authors reported a pathological complete response rate of 25 % among the cohort who had received paclitaxel, compared to 10 % among those who did not, with the difference being statistically significant (p = 0.012). Additionally, the authors reported higher median overall and progression-free survival rates among the group of women who received FAC followed by paclitaxel. Table 15.1 summarizes selected studies of primary systemic chemotherapy for IBC.
Table 15.1
Selected studies of primary systemic chemotherapy for inflammatory breast cancer patients
Author/year of publication | No. of patients | Regimen of chemotherapy | Clinical response rate | 5-year overall survival |
|---|---|---|---|---|
Ueno et al. 1997 [52] | 178 | Anthracycline based | 71 % | 40 % |
Cristofanilli et al. 2001 [56] | 240 | FAC vs. FAC + Pac | 74 % vs. 82 % | NA |
Harris et al. 2003 [57] | 54 | CMF or CAF | 52 % | 56 % |
Low et al. 2004 [58] | 46 | CAF-M | 57 % | 26.7 % (10 years) |
Baldini et al. 2004 [55] | 68 | CEF or CAF | 73.6 % | 44 % |
Veyret et al. 2006 [59] | 120 | FEC-HD | 91.1 % | 41.2 % (10 years) |
Targeted Therapy
The introduction of the humanized monoclonal antibody, trastuzumab, has revolutionized the management of women with breast tumors that overexpress HER2 positively, impacting survival in both early- and advanced-stage breast cancers [56, 60, 61]. Furthermore, the incorporation of trastuzumab into preoperative regimens has been shown to increase pathological complete response rates [62]. With a high incidence of HER2 overexpression in IBC [63], the efficacy of trastuzumab among these women has been explored. Several small prospective studies have explored the incorporation of trastuzumab into the preoperative systemic chemotherapy regimen among women with IBC [64–66]. These studies have reported pathological complete response rates ranging from 17 to 40 %. More recently, Gianni et al. [67] reported on a phase III, randomized clinical trial that included 327 women with HER2-positive locally advanced breast cancer, 27 % of whom had IBC. The objective of the study was to evaluate the efficacy of the addition of trastuzumab to an anthracycline- and taxane-based preoperative regimen. The authors reported that the addition of trastuzumab increased the 3-year event-free survival from 53.3 to 70.1 % (p = 0.0007).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree