Inflammation
Sahdeo Prasad
Bharat B. Aggarwal
INTRODUCTION
Extensive research over the last half a century indicates that inflammation plays an important role in cancer. Although acute inflammation can play a therapeutic role, low-level chronic inflammation can promote cancer. Different inflammatory cells, the various cell signaling pathways that lead to inflammation, and biomarkers of inflammation have now been well defined. These inflammatory pathways, which are primarily mediated through the transcription factors nuclear factor kappa B (NF-κB) and signal transducer and activator of transcription 3 (STAT3), have been linked to cellular transformation, tumor survival, proliferation, invasion, angiogenesis, and metastasis of cancer. These pathways have also now been linked with chemoresistance and radioresistance. This chapter considers the role of inflammation in cancer and its potential for cancer prevention and treatment.
Inflammation is the complex biologic responses of the body to irritation, injury, or infection. The recognition of inflammation dates back to antiquity. As documented by Aulus Cornelius Celsus, a Roman of the 1st century AD, inflammation is characterized by the tissue response to injury that results in rubor (redness, due to hyperemia), tumor (swelling, caused by increased permeability of the microvasculature and leakage of protein into the interstitial space), calor (heat, associated with increased blood flow and the metabolic activity of the cellular mediators of inflammation), and dolor (pain, in part due to changes in the perivasculature and associated nerve endings). Rudolf Virchow subsequently added functio laesa (dysfunction of the organs involved) in the 1850s. The process includes increased blood flow with an influx of white blood cells and other chemical substances that facilitate healing. Inflammation is also considered the body’s self-protective attempt to remove harmful stimuli, including damaged cells, irritants, or pathogens, and to begin the healing process.
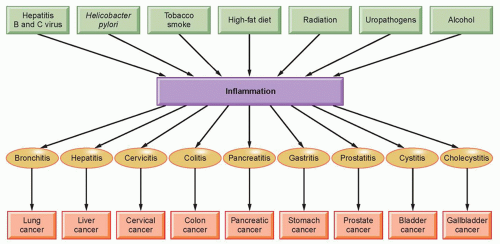
The word inflammation is derived from the Latin inflammo (meaning “I set alight, I ignite”). Because inflammation is a stereotyped response, it is considered a mechanism of innate immunity, as compared with adaptive immunity. On the basis of longevity, inflammation is classified as acute or chronic. When inflammation is short term, usually appearing within a few minutes or hours and ceasing upon the removal of the injurious stimulus, it is called acute. However, if it persists longer, it is called chronic inflammation, which leads to simultaneous destruction from the inflammatory process. Inflammation is beneficial when it is acute; however, chronic inflammation leads to several diseases, including cancer. Cancer is primarily a disease of lifestyle, with 30% of all cancers having been linked to smoking, 35% to diet, 14% to 20% to obesity, 18% to infection, and 7% to environmental pollution and radiation (Fig. 6.1).1 Smoking, obesity, infections, pollution, and radiation are all known to activate proinflammatory pathways.2 Therefore, understanding how inflammation contributes to cancer etiology is important for both cancer prevention and treatment.3
MOLECULAR BASIS OF INFLAMMATION
Although it is clear that inflammation and cancer are closely related, the mechanisms underlying persistent and chronic inflammation in chronic diseases remain unclear. Numerous cytokines have been linked with inflammation, including tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, IL-8, IL-17, and vascular endothelial growth factor (VEGF). Among various cytokines that have been linked with inflammation, TNF is a primary mediator of inflammation linked to cancer.4 However, it has been shown that proinflammatory transcriptional factors (activator protein [AP]-1, STAT3, NF-κB, hypoxia-inducible factor [HIF]-1, and β-catenin/Wnt) are ubiquitously expressed and control numerous physiologic processes, including development, differentiation, immunity, and metabolism in chronic diseases. Although these transcription factors are regulated by completely different signaling mechanisms, they are activated in response to various stimuli, including stresses and cytokines, and are involved in inflammation-induced tumor development and its metastasis.5 Interestingly, inflammation plays a role at all stages of tumor development: initiation, progression, and metastasis.2 In initiation, inflammation induces the release of a variety of cytokines and chemokines that promote the release of inflammatory cells and associated factors. This further causes oxidative damage, DNA mutations, and other changes in the tissue microenvironment, making it more conducive to cell transformation, increased survival, and proliferation. Inflammation also contributes to tissue injury, remodeling of the extracellular matrix, angiogenesis, and fibrosis in diverse target tissues. Among all the inflammatory cell signaling pathways, NF-κB has been shown to play a major role in cancer,6,7 and TNF is one of the most potent activators of NF-κB.8,9
ROLE OF INFLAMMATION IN TRANSFORMATION
Transformation is the process by which the cellular and molecular makeup of a cell is altered as it becomes malignant. Numerous factors are involved in the process of cell transformation, including inflammation. A clinical study has shown that chronic inflammation due to heavy metal deposition in lymph nodes leads to malignant transformation and, finally, to patient death.10 More recently, chronic exposure to cigarette smoke extract11 and arsenite12 has been shown to induce inflammation followed by epithelial-mesenchymal transition and transformation of human bronchial epithelial (HBE) cells. Furthermore, activation of NF-κB and HIF-2α increased the levels of the proinflammatory IL-6, IL-8, and IL-1β, which are essential for the malignant progression of transformed HBE cells. Sox2, another important molecular factor, cooperates with inflammation-mediated STAT3 activation, which precedes the malignant transformation of foregut basal progenitor cells.13 A clinical study reported that the p53 mutation is a critical event for the malignant transformation of sinonasal inverted papilloma. This p53 mutation resulted in cyclooxygenase (COX)-2-mediated inflammatory signals that contribute to the proliferation
of advanced sinonasal inverted papilloma.14 In another study in patients, the YKL-40 protein was found to be involved in chronic inflammation and oncogenic transformation of human breast tissues.15 Inflammation-mediated transformation was also found to be regulated by MyD88 in a mouse model through Ras signaling.16 In addition, inflammation contributed to the activation of the epidermal growth factor receptor (EGFR) and its subsequent interaction with PKCδ, which leads to the transformation of normal esophageal epithelia to squamous cell carcinoma.17 Activation of Src oncoprotein triggers an inflammatory response mediated by NF-κB that directly activates Lin28 transcription and rapidly reduces let-7 microRNA levels. The inflammatory cytokine IL-6 mediates the activation of STAT3 transcription factor, which results in the transformation of cells.18
of advanced sinonasal inverted papilloma.14 In another study in patients, the YKL-40 protein was found to be involved in chronic inflammation and oncogenic transformation of human breast tissues.15 Inflammation-mediated transformation was also found to be regulated by MyD88 in a mouse model through Ras signaling.16 In addition, inflammation contributed to the activation of the epidermal growth factor receptor (EGFR) and its subsequent interaction with PKCδ, which leads to the transformation of normal esophageal epithelia to squamous cell carcinoma.17 Activation of Src oncoprotein triggers an inflammatory response mediated by NF-κB that directly activates Lin28 transcription and rapidly reduces let-7 microRNA levels. The inflammatory cytokine IL-6 mediates the activation of STAT3 transcription factor, which results in the transformation of cells.18
ROLE OF INFLAMMATION IN SURVIVAL
Numerous findings across different cancer populations have suggested that inflammation has an important role in carcinogenesis and disease progression.19,20 The important markers of systemic inflammatory response in both in vitro findings and clinical outcomes include plasma C-reactive protein (CRP) concentration,21,22 hypoalbuminemia,23 and the Glasgow Prognostic Score (GPS), which combines CRP and albumin.24,25 In addition to these, hematologic markers of systemic inflammatory response such as absolute white-cell count or its components (neutrophils, neutrophil-tolymphocyte ratio [NLR]),26,27,28 platelets, and a platelet-to-lymphocyte ratio29,30 are also prognostic indicators for cancer clinical outcomes. Whether these inflammatory biomarkers influence the survival of cancer patients is discussed in this section.
In a study of 416 patients with renal cell carcinoma, with 362 patients included in the analysis, elevated neutrophil count, elevated platelet counts, and a high NLR were found. This inflammatory response was predictive for shorter overall patient survival.31 Another study in unresectable malignant biliary obstruction (UMBO) found that patients with low GPS (0 and 1) had better postoperative survivals than did patients with a higher GPS. The 6-month and 1-year survival rates were 58.1% to 27.3%, respectively, for patients with low GPS and 25% to 6.2%, respectively, for patients with a higher GPS.32 It has been also shown that prostate cancer patients with aggressive, clinically significant disease and an elevated GPS2 had a higher risk of death overall as well as highgrade disease.33 Other than GPS, age and gastrectomy have also been shown to independently influence the disease-specific and progression-free survival of gastric cancer patients.34 A biomarker of systemic inflammation, the blood NLR, predicted patient survival with hepatocellular carcinoma (HCC) after transarterial chemoembolization. Patients in whom the NLR remained stable or became normalized after transarterial chemoembolization showed improved overall survival compared with patients showing a persistently abnormal index of NLR.35
A further study found that inflammatory transcription factors and cytokines contribute to the overall survival of patients. One study found that 97% of patients with epithelial tumors of malignant pleural mesothelioma and 95% of patients with nonepithelial tumors expressed IL-4Rα protein, and this strong IL-4Rα expression was correlated with a worse survival. In response to IL-4, human malignant pleural mesothelioma cells showed increased STAT6 phosphorylation and increased production of IL-6, IL-8, and VEGF without any effect on proliferation or apoptosis. This finding indicates that high expression of STAT6 as well as STAT3 and cytokines is inversely correlated with survival in patients.36,37 NF-κB, along with IL-6, contributes to the survival of mammospheres in culture, because NF-κB and IL-6 were hyperactive in breast cancer-derived mammospheres.38 In addition, elevated CRP and serum amyloid A (SAA) were associated with reduced disease-free survival of breast cancer patients.39 In gastroesophageal cancer, proinflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α and acute phase protein concentrations (CRP) were found to be elevated, and these levels were associated with reduced survival of patients.40 Additionally, the Bcl-2 family protein COX-2, which is regulated by inflammatory transcription factors, is also involved in the survival of cancer cells.41,42 Thus, we conclude that inflammation in general contributes to poor survival of patients.
In contrast to these findings, an in vivo study of dogs with osteosarcoma showed that survival improvement was apparent with inflammation or lymphocyte-infiltration scores >1, as well as in dogs that had apoptosis scores in the top 50th percentile.43 Also, in patients with epithelioid malignant pleural mesothelioma, a high degree of chronic inflammatory cell infiltration in the stromal component was associated with improved overall survival.44
ROLE OF INFLAMMATION IN PROLIFERATION
Several studies have shown that cell proliferation is affected by inflammation.45 More significantly, proliferation in the setting of chronic inflammation predisposes humans to carcinoma in the esophagus, stomach, colon, liver, and urinary bladder.46 In postgastrectomy patients, Helicobacter pylori induced inflammation and was associated with increased epithelial cell proliferation.47 Even in the mouse model, chronic infection with Helicobacter hepaticus induced hepatic inflammation, which further led to hepatic cell proliferation.48 Other reports found an increased expression of the cell proliferative markers PCNA and Ki-67 in the linings of inflamed odontogenic keratocysts compared with noninflamed lesions.49,50 These findings suggest the existence of greater proliferative activity in the cells with inflammation. Wang et al.51 showed an increased expression of cell proliferative markers PCNA and Ki-67 in a sample of 45 patients with benign prostatic hyperplasia.
The inflammatory biomarker COX-2 was also associated with the proliferation of cells. The highest proliferation index was found in COX-2-positive epithelium.51 The association of COX-2 and proliferation was also reported in a rat model. The carcinogen dimethylhydrazine (DMH) induces an increase in epithelial cell proliferation and in the expression of COX-2 in the colon of rats.52 Erbb2, a kinase, regulates inflammation through the induction of NF-κB, Comp1, IL-1β, COX-2, and multiple chemokines in the skin by ultraviolet (UV) exposure. This inflammation has been shown to increase the proliferation of skin tissue after UV irradiation.53
ROLE OF INFLAMMATION IN INVASION
A characteristic of invasive cancer cells is survival and growth under nonadhesive conditions. This invasion of cancer cells causes the disease to spread, which results in poor patient survival.54 A strong relationship has been documented between inflammation and cancer cell invasion.55,56 In a study of 150 patients with HCC, a high GPS score was associated with a high vascular invasion of cancer cells.57 Another study of colorectal cancer also supports the links between inflammation and the invasion of cancer cells, with a finding that a high GPS increased the invasion of colorectal cancer cells.58 In patients with esophageal squamous cell carcinoma, a high GPS score also showed a close relationship with lymphatic and venous invasion.59
At the molecular level, various proteins are known to be involved in tumor cell invasion. MMP-9, a gelatinase that degrades type IV collagen—the major structural protein component in the extracellular matrix and basement membrane—is thought to play an important role in facilitating tumor invasion, as it is highly expressed in various malignant tumors.60,61 Additionally, the high expression of HIF-1α has been proposed as being associated with a greater incidence of vascular invasion of HCC. This expression of HIF-1α was further correlated with high expression of the inflammatory molecule COX-2.62
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree