EPIDEMIOLOGY
Heart failure compromises the health of >5.7 million Americans. About 670,000 new episodes are diagnosed each year, and 282,000 persons die because of heart failure annually. The attributable financial impact of the treatment of heart failure is estimated at >39 million dollars. While the incidence and prevalence of heart failure continue to increase, the donor pool has remained static over time. The 2,000 heart transplants performed each year contrast with the 3,000 patients awaiting transplant at any given time (
60,
61).
The introduction of the LVAD catapulted the management of severe end-stage cardiomyopathy refractory to inotropic therapy, intra-aortic balloon counterpulsation, or both, into a new era. LVADs were originally approved in 1994 by the Food and Drug Administration (FDA) as a bridge to transplantation. Subsequent studies demonstrated that the use of the LVAD was associated with improvement in hemodynamic and end-organ function and conferred a meaningful survival benefit in implanted patients as compared with controls managed with medical therapy alone. An impressive 70% of patients survived until heart transplantation (
62,
63). Furthermore, following transplantation, the survival at 3 years was 95% ± 4% for the LVAD group and 65% ± 10% years for the control inotropesalone group (
64). Even in the presence of LVAD-associated infections, heart transplantation recipients had similar outcomes, including long-term survival, when compared with patients without LVAD infections (
65,
66,
67), although there was a doubling of LVAD-support days that delayed transplantation with a trend toward longer hospital stays posttransplant and increased early mortality resulting from a newly acquired infection in the cohort with LVAD-related infection (
68,
69). These important observations quieted the unease of subjecting LVAD-associated infected patients to intense immunosuppression following their transplants for fear of aggravating their infections (
68).
When it became apparent that patients managed with the LVAD had better outcomes compared to their medically treated counterparts, the indications for LVAD implantation broadened to include those ineligible for transplantation (destination therapy). The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial investigated the use of LVADs for destination therapy (
63). In LVAD recipients, sepsis from any cause accounted for 41% of deaths, whereas the mortality was 17% in patients without sepsis. Within 3 months after implantation, the probability of an HAI related to the LVAD was 28%. The Kaplan-Meier survival analysis did show a 48% reduction in the risk of death from any cause in those patients randomized to LVAD implantation during the first year. However, the aggregate adverse event rate was twice as likely to occur in LVAD patients. By the second year of study, the survival rate of 23% between the two groups was not statistically significant. The LVAD recipients who did not develop sepsis had superior survival rates of 60% at 1 year and 38% at 2 years compared to 39% and 8%, respectively, in LVAD patients who developed sepsis. Localized infections, such as percutaneous site or pocket infection, did not have an adverse impact on survival (
71). An additional 2 years of observation in the REMATCH trial revealed that patients randomized to LVAD implantation in the period after 2000 had a statistically significantly higher survival rate of 59% at 1 year and 38% at 2 years when compared to the 44% and 21% rates, respectively, for the medically treated group. The improved survival rate in patients implanted during the second study period was attributed to the experience gained in areas of patient care and device modifications (
72). The current survival rates with the continuous-flow LVADs for the bridge-to-transplant population is at least 80% at 1 year and 70% at 2 years (
72).
The LVAD infection attack rate is estimated to be around 30% (range, 16% to 37%) and likely reflects the population and device studied (
73,
74,
75). A review of 46 patients with LVAD-associated infections (the most common being the driveline site) noted that infections developed at an average of 65 days postimplantation with a mortality rate of 17% (eight patients) with (five of eight) infected patients dying from sepsis before transplantation (
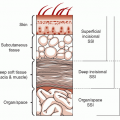
76). Postoperative LVAD-associated infections were identified in 46% of 35 patients in whom 36 LVADs were implanted for a mean of 73 days. Deep SSIs were associated with the requirement for postoperative hemodialysis (
77). Zierer reviewed the first-generation LVAD late-onset driveline infections between 1995 and 2005. Late driveline infections developed in 17 or 23% of patients that occurred at a median of 158 days after implantation. Although the number and duration of readmissions to the hospital greatly increased, there was a nonstatistical decrease in survival, 41% versus 70% at 5 years (
78).
Refinement in the design and technology of LVADs led to the introduction of the second-generation, continuous-flow devices that include the HeartMate II (Thoractec), MicroMed DeBakey (MicroMed), Jarvik 2000 Heart (Jarvik Heart), and VentrAssist (Ventracor). With an improved design that includes a more compact device that is simpler with fewer moving parts, a smaller percutaneous driveline, and that has eliminated the use of polyurethane membranes or PVs that would increase the risk of infections, along with the experience gained with time, the recipients of the continuous-flow LVADs had significantly better survival rates and lower incidence of infectious complications compared to those who had received the first-generation pulsatile devices.
Despite improvement in survival advantage and durability of the second-generation continuous-flow LVADs, infection and sepsis continue to be a major complication that occur in patients undergoing implantation as a bridge to transplantation and for destination therapy.
In the HeartMate II bridge-to-transplantation trial, a significant proportion of patients had local non-LVAD infections (28%), sepsis (20%), or LVAD driveline infections within 1 year (14%) after LVAD implantation (
79). More recently, the HeartMate II destination therapy trial has demonstrated even higher rates of infection, including local non-LVAD infections (49%), sepsis (36%), and LVAD-related infections (35%) in the HeartMate II therapy arm (
80). Even so, this study also concluded that the HeartMate II had better survival rates, lower incidences of LVAD-related infections, local non-LVAD-related infections, and sepsis compared to its earlier counterpart. Topkara reported his experience with 81 patients who underwent implantation of the second-generation (continuous-flow) LVADs. Forty-two (51.9%) patients developed at least one type of infection. Additionally, the patients who developed sepsis had increased mortality (61.9% septic vs. 18% nonseptic patients) at 2 years, whereas patients who developed driveline or pocket infections had no effect on survival, although all infections resulted in a significantly prolonged hospital stay and a trend toward increased mortality (
81).
The national database, the Interagency Registry for Mechanical Circulatory Support (INTERMACS), reported data on 1,158 LVADs that were implanted between June 2006 and March 2009. There were 1,092 primary implants and 66 nonprimary implants, of which 564 were second-generation devices. The survival rates of 83% at 6 months, 74% at 1 year, and 55% at 2 years were reported. Infections following implantation developed in 16% of patients with an overall rate of 17.46 infections per 100 patient-months during the first 12 months after implant. Beyond the first 30 days after implantation, infection was second to heart failure as the most common cause of death in this cohort of patients, with rates of 12.9%, 17.4%, and 15.4% of deaths (
N = 122) occurring from infections in the bridge-to-transplantation, bridge-tocandidacy, and destination patients, respectively. There was a significant decrease (
p < .0001) in infectious complications, comparing the first-generation pulsatile LVAD (28.9 infectious complications/100 months of device use, 406 patients) and the second-generation continuous-flow LVAD (11.8/100 months of device use, 548 patients) (
82). Other investigators also have concluded that there was a reduction in LVAD-associated and nondevice-associated infections in patients ineligible for transplantation (
80,
83,
84).
A report on healthcare-associated LVAD-associated BSIs in 214 patients revealed an incidence of 38%; the BSI was statistically significantly associated with death (the overall incidence of BSI in recipients of LVADs from any cause was 49%). Fungemia had the highest hazard ratio (10.9) followed by gram-negative (with
Pseudomonas aeruginosa predominating) and gram-positive bacteremia. The duration of LVAD support before the onset of any BSI was 19.5 days for gram-negative bacilli, 28 days for yeast, and 242 days for gram-positive cocci (
69). Forty-six LVAD-associated infections were described in 50% (38/76) of patients who underwent LVAD implantation as a bridge to transplantation. Twenty-nine LVAD-associated BSIs included five episodes of LVAD endocarditis and 17 localized LVAD infection (i.e., exit site, LVAD pocket infections) (
68). In a study of 109 consecutive patients supported by LVADs as a bridge to transplant, 65 patients (60%) during 584 ± 389 device-days developed a BSI that resulted in a significant adverse impact on survival after LVAD implantation. The risk factors associated with death included postoperative right heart failure and BSIs caused by pathogens other than gram-positive cocci. The investigators concluded that urgent cardiac transplantation should be considered in these patients. None of the 22 patients who were transplanted had a recurrence of their BSI and all were alive at 3 years posttransplant (
85).
MICROBIOLOGY
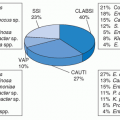
The microbiology of LVAD-associated infections is fairly consistent with gram-positive organisms predominating and likely resulting from the disruption of the cutaneous barrier with the subsequent biofilm formation, followed by
P. aeruginosa and enteric gram-negative rods. CONS was the most frequent pathogen isolated in BSIs in LVAD-implanted patients from any cause, followed by
S. aureus (of which 36% were methicillin-resistant [MRSA]),
Candida sp., and
P. aeruginosa. Although the enterococci accounted for only about 8% of BSIs, 50% of the isolates were vancomycin-resistant (
69). A more recent review reported that of 221 BSIs that occurred in 65 patients, the majority was caused by gram-positive cocci (159 or 72%), with 101 caused by
S. aureus (MRSA, 65 or 29%; methicillin-susceptible [MSSA], 36 or 16%) and 50 or 23% caused by CONS followed by gram-negative rods (17%) and fungi (6%) (
85). Of the 300 patients who received a VAD, 108 (36%) developed VAD infection, including 85 bacterial and 23 fungal infections. The most common
bacterial causes of infection were
S. aureus, CONS enterococci, and
P. aeuruginosa. The most common fungal etiologic agent was
C. albicans. Only the use of total parenteral nutrition was associated with the development of a fungal VAD infection in multivariate analyses (odds ratio, 6.95; 95% confidence interval, 1.71 to 28.16;
p = .007). Patients who experienced a fungal VAD infection were less likely to be cured (17.4% vs. 56.3%;
p = .001) and had greater mortality (91% vs. 61%;
p = .006), compared with those who experienced a bacterial VAD infection (
75).
Of 47 isolates from 76 LVAD patients who developed LVAD-associated infections, 78% and 19% of LVAD-associated infections were due to gram-positive organisms and gram-negative rods, respectively, with only one infection due to yeast. Diabetes mellitus was identified as a risk factor for the 30 BSIs in this cohort. There was a striking incidence of posttransplantation-invasive vancomycin-resistant
Enterococcus faecium (VREF) infections in six patients with an associated mortality of 67%. This is in marked contrast to LVAD-support patients who did not develop LVAD-associated infections, and there were no postoperation-invasive VREF infections (
68). Resistant
Staphylococcus and
P. aeruginosa were the most common pathogens leading to device- and nondevice-related local infections (
81).