| Preoperative period | Intraoperative period | Postoperative period |
|---|---|---|
| Lack of pathogen-specific immunity Severity of underlying clinical illnesses and comorbidity Fulminant hepatic failure Renal insufficiency Anemia Prior fungal infection (i.e., endemic mycoses and aspergillosis) | Presence of pathogens in the transplant allograft (donor-derived infections) Prolonged operative time Complicated surgical procedure Profound blood loss and infusion of large volume of blood products Choleduchojejunostomy (liver recipients) | Prolonged hospitalization Prolonged duration of stay in intensive care unit Prolonged antibiotic use (fungal infections and Clostridium difficile) Renal insufficiency Gastrointestinal and biliary complications Vascular complications Anastomotic leaks Wound dehiscence Lymphocyte-depleting drugs, high-dose steroid use, and treatment of allograft rejection Immunosuppresive drugs CMV and HHV-6 reactivation Reoperation within 1 month post-transplantation Retransplantation |
Abbreviations: HHV = human herpesvirus; CMV = cytomegalovirus.
The transplant recipient
The epidemiologic exposures of potential transplant recipients should be assessed to determine the risk of infection and guide preventive measures. Table 89.2 lists the recommended screening tests in the evaluation of potential recipients (and their donors) prior to transplantation. Some candidates will be found to have active infection; these infections do not generally preclude transplantation, but they should be adequately controlled and treated prior to and after the transplant procedure.
| Human immunodeficiency virus (HIV) antibody |
| Herpes simplex virus (HSV) 1 and 2 antibody |
| Cytomegalovirus (CMV-IgG) antibody |
| Epstein–Barr virus (EBV) antibody panel |
| Varicella-zoster virus (VZV) antibody |
| Toxoplasma antibody (in heart recipients) |
| Rapid plasma reagin test and treponemal tests for syphilis |
| Human T-cell lymphotropic virus (HTLV) I and II antibody (selected cases only) |
| Hepatitis C virus (anti-HCV) antibody |
| Hepatitis B virus (HBV) surface antigen |
| HBV surface antibody |
| HBV core immunoglobulin (IgM) and IgG antibody |
| PPD skin testing or interferon-gamma release assay (e.g., QuantiFERON TB test) |
| Strongyloides stercoralis serology (with stool ova and parasites for candidates from endemic areas) |
| Coccidioides immitis serology (for candidates from endemic areas) |
| Trypanosoma cruzi serology (for donors and recipients from endemic areas) |
| West Nile virus (nucleic acid testing for living donors) |
The transplant donor
The epidemiologic exposures of transplant donors should be determined so that the potential for donor-derived infections is reduced. Screening for cytomegalovirus (CMV), Epstein–Barr virus (EBV), Toxoplasma gondii (for heart transplant recipients), hepatitis B and C viruses, and human immunodeficiency virus (HIV) are routinely performed in transplant donors (Table 89.2). Transplant donors often have prolonged stay in the hospital prior to organ harvest, and they may have acquired nosocomial infections (Table 89.3) which could be transmitted to the transplant recipient through transplantation. Occurrence of donor-derived infections should be reported to the national database.
| Community-acquired infections | |
| Residence in endemic areas Exposure to index cases Ingestion of contaminated water and food Environmental source Vector-borne | Mycobacterium tuberculosis, Strongyloides stercoralis, Blastomyces dermatitidis, Histoplasma capsulatum, Coccidioides immitis, Trypanosoma cruzi, human herpesvirus 8, Plasmodium species, dengue virus Mycobacterium tuberculosis, respiratory viruses (influenza, parainfluenza, respiratory syncytial virus, adenoviruses, SARS) Salmonella species, Campylobacter jejuni, Listeria monocytogenes, Giardia lamblia, Cryptosporidium parvum Aspergillus fumigatus, Nocardia asteroides, Sporothrix schenkii, norovirus West Nile virus, tick-borne diseases, Plasmodium species |
| Nosocomial infections | |
| Contaminated air Contaminated water Hand contact | Aspergillus fumigatus Legionella pneumophila Methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, drug-resistant gram-negative bacilli, influenza virus |
Abbreviation: SARS = Severe acute respiratory syndrome.
The environment
The healthcare environment is a major source of pathogens that cause infectious disease in transplant recipients, including invasive procedures, such as the insertion of indwelling urinary catheters, intravascular catheters, and endotracheal tubes; administration of blood products; and surgical procedures.
Many infections are acquired by the transplant recipients from the community, where natural transmission of pathogens continually occurs. Table 89.3 lists the epidemiologic exposures and the pathogens associated with the specific exposure.
Net state of immunosuppression
The two major factors that influence the overall net state of immunosuppression are: (1) the intensity of pharmacologic immunosuppression and (2) the reactivation of immunomodulating viruses. There is increasing evidence that inherent defects in innate and adaptive immunity may augment the net state of immune deficiency, further increasing the risk of infectious complications after transplantation. The use of immunosuppressive drugs is essential to maintain allograft survival (by preventing acute and chronic graft rejection among solid organ transplant [SOT] recipients) and to prevent graft-versus-host disease (in allogeneic hematopoietic stem cell transplant [HSCT] recipients). The degree of drug-induced immunosuppression is particularly intense during the first 3 months after transplantation, and is characterized by severe impairment of cellular and humoral immunity. Although defect in cell-mediated immunity is a well-recognized effect of the immunosuppressive drugs, impairment in humoral immunity, as indicated by severe hypogammaglobulinemia, may also occur. As a result, the use of immunosuppressive drugs (e.g., mycophenolate mofetil [MMF], prednisone, tacrolimus, cyclosporine, alemtuzumab, among others) places the patients at very high risk of infectious complications. For example, lymphocyte-depleting agents such as OKT3 monoclonal antibody and antithymocyte globulin increase the risk of CMV disease and other opportunistic infections such as human herpesvirus 6 (HHV-6), Aspergillus spp., and Pneumocystis jirovecii (carinii). These drugs could accelerate the clinical course of post-transplant hepatitis C virus (HCV) infection. Severe hypogammaglobulinemia after transplantation may increase the risk of infections with encapsulated organisms such as Streptococcus pneumonia. The reactivation of viruses with immunomodulating properties, such as CMV and HHV-6, during periods of intense drug-induced immunosuppression may paradoxically further enhance the overall state of immunosuppression. CMV and HHV-6 have been associated with an increased risk of bacterial and fungal opportunistic infections. The negative effect of CMV and HHV-6 on the course of post-transplant HCV infection is also well described.
Time course of infections after transplantation
Infections after transplantation follow a stereotyped temporal pattern. Figures 89.1 and 89.2 depict the timing of infections after SOT (Figure 89.1) and allogeneic HSCT (Figure 89.2). However, the natural history of these infections is evolving, as influenced by various factors, most notably the use of antimicrobial prophylaxis. For example, CMV disease traditionally occurs during the first 3 months after transplantation, but this has been delayed among high-risk CMV donor-positive/recipient-negative patients to the first 3 months after completion of antiviral prophylaxis. In addition, use of antimicrobial prophylaxis has modified the drug susceptibilities of various pathogens, as exemplified by the emergence of fluconazole-resistant Candida spp. infections in centers utilizing fluconazole prophylaxis. There is also increasing incidence of drug resistance among bacterial pathogens, which could complicate antimicrobial prophylaxis and treatment approaches.
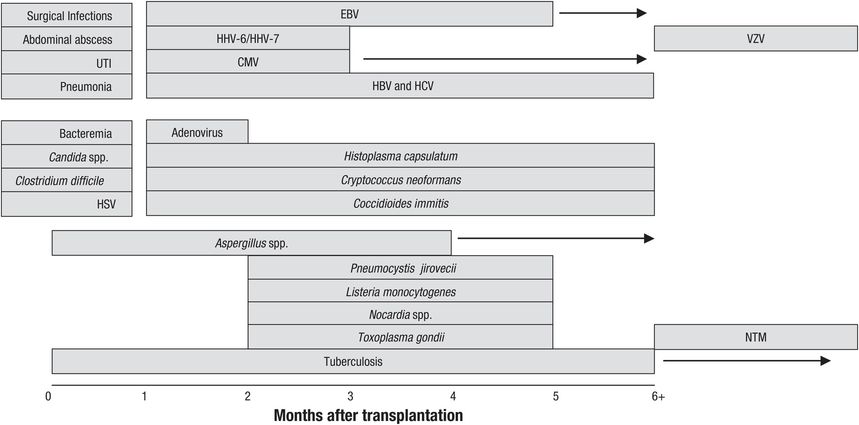
Figure 89.1 Natural history timeline of infections following solid organ transplantation in the absence of antimicrobial prophylaxis.
Abbreviations: HSV = herpes simplex virus; CMV = cytomegalovirus; EBV = Epstein–Barr virus; HHV = human herpesvirus; VZV = varicella-zoster virus; HBV = hepatitis B virus; HCV = hepatitis C virus; UTI = urinary tract infection; NTM = nontuberculous mycobacteria.
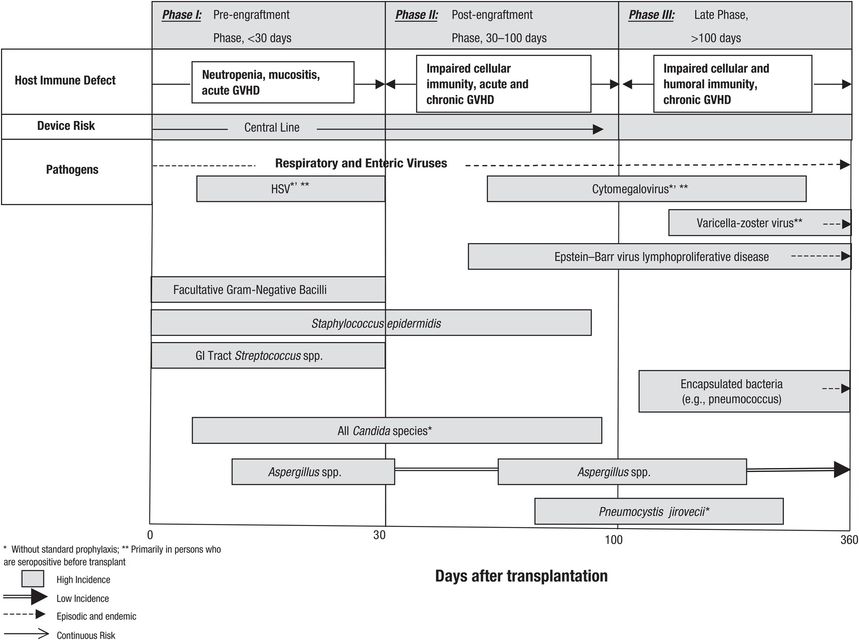
Figure 89.2 Natural history timeline of infections following allogeneic hematopoietic stem cell transplantation in the absence of antimicrobial prophylaxis. HSV = herpes simplex virus; GVHD = graft-versus-host disease; GI = gastrointestinal.
Timetable of infections after solid organ transplantation
Infections after SOT follow a characteristic time frame that reflects the net state of immunosuppression. These time frames are important to remember during the clinical evaluation of patients presenting with various clinical syndromes after transplantation. In this regard, clinical syndromes such as pneumonia and cellulitis may occur at any time, but the offending pathogen may vary depending on its onset after transplantation.
The first month after SOT
The three major sources of infections during this period are (1) infection that is present in the recipient prior to transplantation (i.e., bacterial peritonitis in liver recipients, catheter-related bloodstream infection in kidney recipients, bacterial pneumonia in lung recipients, and infected cardiac device in heart transplant recipients); (2) infection transmitted in the allograft (e.g., unrecognized or undiagnosed bacterial, viral, and fungal infection prior to organ harvest); and (3) infections related to surgery and hospitalization.
The majority of infections that occur during this period are related to surgical procedures and hospitalization (Figure 89.1): surgical site infections due to Staphylococcus spp. and Streptococcus spp. or other nosocomially acquired pathogens; catheter-associated urinary tract infections with gram-negative bacteria such as Escherichia coli, gram-positive bacteria such as enterococcus, and fungi such as Candida albicans; nosocomial and ventilator-associated pneumonia due to drug-resistant Pseudomonas aeruginosa, Acinetobacter spp., Staphylococcus aureus, and others; and catheter-associated bloodstream infection with gram-positive bacteria such as S. aureus, enterococcus, and coagulase-negative staphylococcus are seen. Intra-abdominal infections are especially common among liver recipients who require abdominal re-exploration (for hepatic artery thrombosis, bleeding, biliary leakage, or retransplantation). Prolonged hospitalization further increases the risk of nosocomial pneumonia, urinary infections, and antibiotic-related Clostridium difficile diarrhea. The widespread use of antibacterial agents for prophylaxis and treatment of defined infections has led to the rising incidence of C. difficile infection during this period.
During this time period, herpes simplex virus (HSV) types 1 and 2 commonly reactivate and may cause localized ulcerative or disseminated disease in the HSV-seropositive SOT recipients; antiviral prophylaxis with acyclovir (or valganciclovir for CMV) has significantly decreased its incidence. Donor-derived infections such as an unrecognized fungal infection (due to Histoplasma capsulatum or Cryptococcus neoformans) and other unusual infections such as West Nile virus (WNV), rabies virus, or lymphocytic choriomeningitis virus may be manifested clinically during this period. One clue to the possible donor-derived infection is the occurrence of similar illness among recipients of organs from the same organ donor.
Second to the sixth month after SOT
This is the period when most opportunistic infections classically occur. During this period, infections due to CMV, EBV, and HHV-6 occur as a result of the severe impairment in cell-mediated immunity. Opportunistic infections with Listeria monocytogenes, Aspergillus fumigatus, and Pneumocystis jirovecii are also a clinical reflection of a severely impaired immune function. In the absence of antiviral prophylaxis, the β-herpesviruses (CMV and HHV-6) reactivate and cause fever and tissue-invasive clinical disease during this period. Invasive aspergillosis, most commonly due to A. fumigatus, may occur during this time, especially among patients transplanted for fulminant hepatic failure and those with epidemiologic exposure (i.e., renal insufficiency, exposure to areas of construction, or previous colonization among lung transplant recipients) and profound immunosuppression. Pneumonia is the most common clinical presentation of aspergillosis, but the clinical illness may disseminate to any body organ system, possibly due to the vasculotropic nature of Aspergillus spp., and cause abscesses in many organs, including the liver and the brain. Infections with endemic fungi (e.g., H. capsulatum and Coccidioides immitis) and C. neoformans may occur during this period. P. jirovecii pneumonia traditionally occurs during this period but prophylaxis with trimethoprim–sulfamethoxazole has made this infection, and those due to Nocardia spp., uncommon during this period.
Beyond the sixth month after SOT
There are generally two types of patients with varying risk of infection during this period: (1) those with good allograft function and minimal immunosuppression, and (2) those with poor allograft function as a result of recurrent rejection or chronic allograft dysfunction. In addition, liver transplant recipients with underlying chronic viral hepatitis (due to hepatitis B or C) may develop an accelerated clinical course characterized by graft failure and the need for retransplantation. These patients may benefit from prophylaxis with hepatitis B immunoglobulin and nucleoside or nucleotide analogs (for hepatitis B patients), or targeted therapy (for hepatitis C patients).
The vast majority of transplant patients will have good allograft function, and their level of immunosuppression has already been reduced to minimal levels. These patients are primarily at risk of infections similar to those observed in nonimmunocompromised populations. However, a small group of SOT patients will have poor graft function as a result of recurrent rejection or chronic dysfunction; these patients are generally considered to be over-immunosuppressed and remain at high risk of opportunistic infections, including those due to P. jirovecii, L. monocytogenes, C. neoformans, Nocardia asteroides, CMV, and Aspergillus spp. infections. Patients with a persistent hypogammaglobulinemia are particularly at risk of pneumonia and bacteremia due to encapsulated organisms.
Infection with endemic mycoses such as H. capsulatum and C. immitis may occur in patients residing in certain geographic regions. One of the most common opportunistic infections during this time is the reactivation of varicella-zoster virus (VZV) causing dermatomal zoster, which has the potential for dissemination. In endemic areas, HHV-8 may reactivate to cause Kaposi’s sarcoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





