Evidence That Environmental Contamination Contributes to Transmission
Table 42-1 provides a summary of the evidence supporting environmental contamination as a source of pathogen transmission. Although much of the evidence is indirect, several recent reports provide substantial evidence of transmission linked to environmental contamination. The evidence is most convincing for pathogens that have a propensity to survive for prolonged periods on dry surfaces, including
C difficile, VRE, MRSA,
Acinetobacter species, norovirus, and
C auris.1
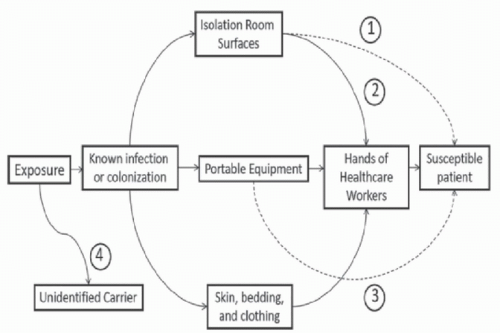
Contamination of Patient Rooms and Portable Equipment Surfaces in rooms of colonized or infected patients frequently become contaminated.
1 The ability to survive on dry surfaces varies for different pathogens. Organisms such as VRE, MRSA,
Acinetobacter species, norovirus, and
C auris can survive for days to weeks on dry surfaces, and
C difficile spores can persist for months.
30 Many studies have demonstrated widespread contamination of surfaces with these pathogens in rooms of colonized patients, although the burden of contamination is generally low.
8 In contrast, organisms such as carbapenem-resistant Enterobacteriaceae survive relatively poorly on dry surfaces and may be recovered less frequently from surfaces in rooms of colonized patients.
31
Portable equipment and other shared items also frequently become contaminated with healthcare-associated pathogens, including
C difficile, MRSA, VRE, multidrugresistant Gram-negative bacilli, and
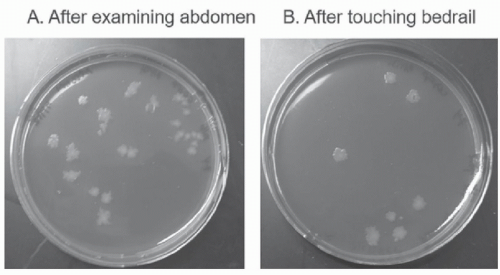
C auris.32 Much of the contamination may occur when equipment is used during medical procedures and patient care activities. In an evaluation of shedding of MRSA by colonized patients during procedures, contamination was detected on 32% of surfaces touched by personnel and on 25% of portable equipment used for the procedures or care activities.
22Transfer From the Environment to Hands of Personnel The hands of HCP are generally considered the primary source of transmission of pathogens in healthcare settings. Several recent studies have demonstrated that contaminated environmental surfaces may be an important source of hand contamination. Hand or glove acquisition of
C difficile spores or MRSA occurred as frequently after contact with commonly touched environmental surfaces in patient rooms as after contact with commonly examined skin sites.
33,34 Figure 42-2 provides an illustration of contamination of gloved hands with
C difficile spores after contact with a
C difficile infection (CDI) patient and with surfaces in the patient’s room. Similarly, hand acquisition of VRE occurred nearly as often after touching the environment in VRE isolation room as after touching the colonized patient and the environment (52% vs 70%, respectively).
35 Several other studies have also demonstrated frequent acquisition of pathogens on hands after contacting contaminated surfaces.
36,37,38 In one study, a positive correlation has been demonstrated between isolation of
C difficile from the hands of personnel caring for CDI patients and the percentage of positive environmental cultures in the CDI patient rooms.
37 Finally, it has been demonstrated that transfer of multidrugresistant bacteria to gloves and gowns of HCP during patient contact increases with environmental contamination.
38
Admission to a Room Previously Occupied by a Colonized Patient Increases the Risk for Subsequent Occupants Environmental surfaces in rooms of patients in contact precautions often remain contaminated after completion of postdischarge cleaning and disinfection.
1 Such contamination is a potential source for pathogen acquisition by subsequent room occupants. Several recent studies have demonstrated that admission to a room previously occupied by a colonized or infected patient increases the risk for subsequent room occupants to acquire the same organism.
6 Organisms associated with increased risk have included
C difficile, MRSA, VRE, and multidrug-resistant Gram-negative bacilli (
Acinetobacter baumannii, extendedspectrum beta-lactamase [ESBL]-producing Gram-negative bacilli, and
Pseudomonas aeruginosa). One prior study found that admission to a room previously occupied by a patient with an ESBL-producing Gram-negative bacillus was not associated with an increased risk for subsequent room occupants after adjusting for colonization pressure and antibiotic exposure in the intensive care unit.
39
Portable Equipment Linked to Pathogen Transmission In several outbreak investigations, contaminated shared medical equipment has been implicated as a potential vector for transmission of healthcare-associated pathogens.
6 The types of equipment linked to transmission have included thermometers, respiratory care equipment, ultrasound probes, pressure transducers, and electrocardiogram leads. For many of these outbreaks, no definitive evidence has linked contaminated equipment to pathogen transmission. Rather, contamination of equipment has been demonstrated, and correction of deficiencies in cleaning and disinfection of equipment has been associated with reductions in colonization or infection with pathogens.
6 However, a recent study that included highly discriminatory molecular typing strongly implicated shared equipment as a vector for transmission of VRE, including as a conduit for transmission between different intensive care unit areas.
40
The devices most convincingly linked to pathogen transmission are shared electronic thermometers. In the 1990s, shared thermometers, including rectal and oral thermometers, were linked to transmission of VRE,
C difficile, and
Enterobacter cloacae.41 In these outbreaks, it was demonstrated that thermometer handles were contaminated, and it was suspected that contamination on the handles was transferred to patients via the hands of personnel. Substitution of single-use disposable thermometers for shared electronic thermometers was associated with significant
reductions in CDI or VRE colonization.
42,43 Based on these findings, guidelines for prevention of CDI in acute care hospitals include a recommendation that single-use disposable thermometers be used in care of CDI patients.
7 In the
E cloacae outbreak attributed to contaminated thermometers, inadequate disinfection practices were identified, and correction of these practices led to control of the outbreak.
41Recently, transmission of the emerging fungal pathogen
C auris has been linked to shared temperature probes.
7 The reusable probes were wiped between patients with quaternary ammonium compound wipes, but it was noted that the probes were difficult to clean and disinfect due to their design with a two-layer rubber sheath protecting the distal end of the wire adjacent to the sensor. The relatively poor activity of quaternary ammonium compounds against
Candida species may have also contributed to inadequate disinfection.
44 Discontinuation of the use of the temperature probes was associated with resolution of an outbreak.
7Benign Surrogate Markers Demonstrate Transmission From Environmental Surfaces Benign surrogate markers, such as nonpathogenic viruses and viral DNA, provide a powerful tool to study routes of pathogen transmission.
45 In several studies, these surrogate markers have demonstrated the potential for contaminated surfaces to serve as a vector for dissemination of microorganisms. In a medical and surgical intensive care unit, it was demonstrated that a viral DNA marker inoculated onto shared portable equipment disseminated widely to surfaces in patient rooms and provider work areas and to other types of portable equipment.
46 In hospital and nursing home units, similar dissemination of viral DNA surrogate markers has been demonstrated from thermometer handles and television remote controls.
47,48
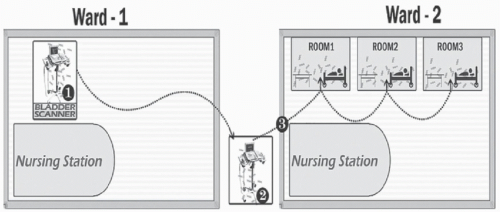
One observation from recent studies using highly discriminatory molecular typing methods is that genetically related organisms are often detected in patients with no shared exposure on the same ward.
49 Benign surrogate markers provide a means to evaluate the potential for personnel and equipment to disseminate pathogens between wards. In an observational study, a viral DNA marker inoculated onto portable equipment on a medical ward disseminated to other wards when equipment was shared.
50 Figure 42-3 provides an illustration of transfer of the marker from a contaminated bladder scanner on one ward to three patient rooms on another ward when the device was borrowed by a nurse from the other ward. These findings highlight the potential for portable equipment to serve as a vector for dissemination of pathogens between wards.