Predisposition of the Elderly to Infection
There are a number of factors that increase the risk of infection in older adults when compared to young adults. The relationships between these risk factors, whether they are comorbidities, waning immunity, or age itself, may be very complex. For example, many older individuals have latent infection with Mycobacterium tuberculosis (i.e., asymptomatic infection) and do not manifest clinical illness despite an aging immune system and the presence of various comorbid conditions. However, superimposing malnutrition, perhaps caused by an intervening stress, may be the last insult necessary to tip the scales toward illness, resulting in clinical manifestations. This complex interplay of risk factors makes it difficult to determine the attributable risk of any one characteristic, and any risk factor in isolation cannot be considered “the cause” of infectious risk in the elderly. However, several well-recognized features associated with advanced age clearly do increase risk for clinical infection; these are reviewed in this chapter.
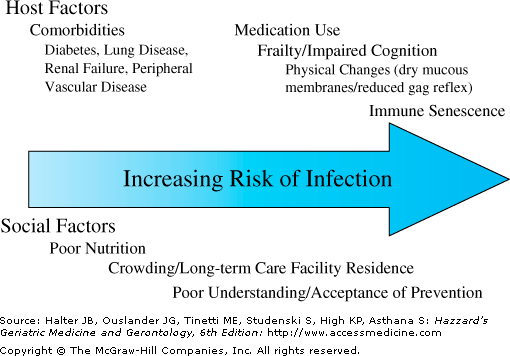
In the elderly individual, the increased incidence of infection and mortality for many infectious diseases (Figure 124-1) is likely a direct result of the comorbid conditions (e.g., diabetes, renal failure, chronic pulmonary disease, edema, immobility) that accompany advanced age. Comorbidity most often results in reduced innate immunity, defined as those responses that are not specific to a given organism or antigen. These include nonspecific barriers such as skin integrity, cough, and mucociliary clearance, and those immune responses triggered by recognition of patterns of microbial products (e.g., endotoxin, lipoteichoic acid) without the need for prior exposure such as complement, tissue phagocytes, and toll-like receptors (TLRs). Perhaps the best clinical example in which comorbidity contributes heavily to infection risk is chronic obstructive pulmonary disease (COPD). This disease, most often caused by prolonged exposure to tobacco smoke, has a high prevalence in older adults. The impaired mucociliary clearance, alveolar macrophage dysfunction, and suppressed cough mechanism that accompany COPD substantially increase the risk for lower respiratory tract infection in the elderly. Comorbid diseases in elderly individuals with infection can also be more important predictors for worse outcomes. For example, community-acquired pneumonia (CAP) is typically treated on an outpatient basis and rarely causes mortality in patients younger than 50 years of age. However, multiple comorbid conditions and advanced age greatly increase the risk of mortality associated with CAP. In fact, while age itself dominates many CAP prognostic indices, advanced age alone is not a predictor of mortality in those persons older than 75 years of age where comorbidity dominates. Furthermore, cognitive decline and other barriers that reduce adherence to medical regimens often necessitate hospitalization of older adults in circumstances where their younger counterparts are often treated as outpatients, further increasing costs and enhancing the rate of complications.
While comorbidities substantially predispose older adults to infection, there are also age-related fundamental changes in the immune response that may predispose the elderly to infection (see Chapter 3), so called immune senescence. Immune senescence is not merely a global state of reduced immunity, but a dysregulation of immune responses at multiple levels. Some aspects of immunity are upregulated, including the inflammatory response, which demonstrates constitutive activation in older adults, as evidenced by elevated C-reactive protein and interleukin (IL)-6 blood levels, and cellular activation of nuclear factor-kappa B (NF-κB). However, other innate immune responses (e.g., natural killer [NK] cell activity) are frequently reduced in older adults, and though originally thought to be normal, more recent data suggest polymorphonuclear neutrophil (PMN) function may also be impaired with reduced microbial phagocytosis and killing.
The most consistent immune deficit identified in advance age is a reduction in adaptive immune responses. There are decreases in naïve T-cell subsets, marked depression of cytokine production (particularly IL-2) and important cellular surface receptors (IL-2 receptor and CD28), and suppression of T-cell responses by inflammatory cytokines that inhibit T cell immunity such as IL-10 and prostaglandin E2.
Although there is little doubt that immune senescence exists, the clinical role of this phenomenon in the predisposition of the elderly person to infection remains uncertain. Many flaws plague the published observational studies, despite herculean efforts to minimize confounding using very strict criteria for entry (in most cases via use of the SENIEUR criteria). Ironically, less than 15% of the aged population meets SENIEUR criteria, and the generalizability and clinical relevance of the accumulated data remain in doubt. Is immune senescence irrelevant in patients with overwhelming comorbidity? Or is it the key factor that shifts the balance toward infection when young adults with comorbidity remain relatively well? A recent superb review highlights the confusing and often contrary results of human studies (see Castle, 2000).
Nutritional status is a major confounder in studies of immunity in the elderly population. Protein–energy malnutrition (PEM) is present in 30% to 60% of patients older than 65 years of age who are admitted to the hospital, and is linked to delayed wound healing, pressure ulcer formation, CAP, increased risk of nosocomial infection, extended lengths of stay, and increased mortality. In community-dwelling older adults, PEM frequently goes unrecognized, but even older adults with mild PEM (i.e., those with a serum albumin of 3–3.5 g/dL) demonstrate poor vaccine responses and diminished cytokine responses to immune stimuli. Despite the strong epidemiologic evidence linking PEM to infection in the elderly, the role of nutritional supplements for preventing infection remains controversial. Some studies of residents who live in long-term care facilities suggest use of supplements may increase caloric intake, but other reports indicate meal-time calorie consumption may reciprocally decrease, leading to no net change in protein or calories. Despite this controversy, there is widespread agreement that adequate nutrition should be encouraged and provided to debilitated elderly individuals, and that adequate nutrition is essential for proper wound healing, and may speed functional recovery from serious infectious diseases (i.e., pneumonia).
Specific micronutrient deficiencies are also common in older adults, and several have been linked to poor immune function (e.g., vitamin B-12 deficiency and inadequate pneumococcal vaccine responses). Several micronutrient supplementation trials have been performed that highlight the potential importance of adequate nutrition even in apparently healthy elderly. Vitamin E supplementation (≥200 mg/day) in healthy elderly individuals enhances delayed-type hypersensitivity (DTH) and vaccine responses for a primary T-cell–dependent antigen (hepatitis B), but has no effect on T-cell-independent vaccine responses (pneumococcal vaccine) or the booster response to tetanus. A word of caution, however: a recent meta-analysis of vitamin E supplementation suggested increased mortality in those taking ≥400 mg/day, highlighting the dangers of megadose therapy with some vitamins (vitamins A, D, E, K). Multivitamin/mineral supplements have been examined in healthy, free-living elderly persons and consistently demonstrate improved serum vitamin levels, but results are inconsistent with regard to preventing clinical infection or boosting vaccine responses. Other studies in elderly residents of long-term care facilities suggest zinc (20 mg/day) plus selenium (100 μg/d), or specific commercial formulas may reduce the risk of infection, but more work is required to define the role of vitamin or mineral supplementation for augmenting immune response in the elderly population, and to determine whether there are specific subpopulations that could help identify target groups for therapy.
There is an increasing recognition that the health of seniors is not only a function of biomedical variables but also socioeconomic status, biophysical environment, and delivery of health care services. This “determinants of health” perspective combines biomedical, behavioral, social, and environmental explanations of illness and health (Figure 124-1). Probably the best example of this with respect to infectious diseases in the elderly individual is that of respiratory tract infections. Population-based studies reveal that lower income is associated with higher rates of CAP and invasive pneumococcal infections among elderly individuals. Lower socioeconomic status may predispose to infection either because of increased exposure to infectious agents (e.g., crowding) or because of increased susceptibility. Although the evidence is limited, this might be a result of worse nutritional status, more frequent exposure to air pollution or tobacco smoke, or inadequate vaccination.
Long-term care residents highlight the concept of “multiple determinants of health,” and the physical environment plays a particularly important role in infections in older adults who reside in long-term care facilities. This subset of the aging population has a particularly high incidence of respiratory and other infections (mainly urinary and skin infections). The close contact residents have with other residents plays a key role in the spread of respiratory infections such as influenza or with bacterial infections such as group A streptococcus (i.e., Streptococcus pyogenes). This combination of frail older individuals in a confined setting can lead to severe outbreaks with high mortality rates. The close proximity of residents, poor adherence to basic infection control measures, and the intense use of antibiotics are factors that lead to the spread of antibiotic-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and multiresistant gram-negative rods. Although the actual infectious threat many of these organisms pose to individual residents is uncertain, the potential reservoir creates a problem when such residents are transferred to acute care hospitals where these organisms can spread to other susceptible hosts.
Diagnosis and Management of Infections in the Elderly Patient
Infectious diseases frequently present with atypical features in older adults. Serious infections may be heralded by nonspecific declines in functional or mental status, or anorexia with decreased oral intake. Underlying illness (e.g., congestive heart failure [CHF] or diabetes) may be exacerbated, leading the elderly patient to seek medical attention. The most common sign that triggers the clinician to look for infection, fever, is often absent in the elderly patient. Several studies show that frail elderly individuals have lower mean baseline body temperatures than the currently accepted normal of 98.6°F (37°C). Animal models of aging demonstrate that temperature elevations in response to endogenous pyrogens (IL-1, IL-6, tumor necrosis factor [TNF]-α) are diminished with advanced age. The decline in basal temperature and blunted response to inflammatory stimuli make it less likely that a frail, older adult will achieve a body temperature commonly recognized as fever. It has been suggested that fever in frail older adults should be defined as (1) persistent elevation of body temperature of at least 2°F (1.1°C) over baseline values, or (2) oral temperatures of 99°F (37.2°C) or greater on repeated measures, or (3) rectal temperatures of 99.5°F (37.5°C) or greater on repeated measures. The sensitivity of detecting fever and infection in the nursing home setting has been improved using this definition, and reasonable specificity maintained. The importance of a “normal” or reduced temperature in the face of significant infection cannot be overemphasized. A blunted febrile response often leads to delayed diagnosis, and is an indicator of a poor prognosis.
Cognitive impairment also heavily contributes to the difficulty in diagnosing infection in the elderly. Subjects are often unable to communicate symptoms and clinicians should have a lower threshold to pursue objective assessments (e.g., laboratory and radiologic evaluations) in cognitively impaired elderly with changes in functional status.
Finally, age- and comorbidity-related changes in anatomy and physiology may confound interpretation of diagnostic evaluations. Perhaps the best example of this is the diminished sensitivity for trans-thoracic echocardiography (TTE) for detecting vegetations on the heart valve in endocarditis. Studies suggest the additional echoes present as a result of age-related calcium deposition reduces sensitivity of TTE from 85% to 90% in adults aged 55 years and younger, to <50% for those older than 70 years of age.
The profound physiologic changes associated with aging and comorbidities alter drug distribution, metabolism, excretion, and interactions (see Chapter 8) Antibiotic dose reductions are occasionally required in the elderly adult because of changes in renal function or predisposition of the elderly adult to important side effects such as changes in mental status (e.g., flouroquinolones). In addition, antibiotic interactions are more frequent because most elderly persons are taking multiple medications. Digoxin, warfarin, oral hypoglycemic agents, theophylline, antacids, and proton pump inhibitors all have significant interactions with commonly prescribed antibiotics. Drug concentrations can increase (e.g., enhanced digoxin toxicity associated with macrolides, tetracyclines, and trimethoprim) or decrease (e.g., reduced quinolone absorption with concomitant administration of antacids) with concomitant drug administration. These changes and the increased incidence of side effects in the elderly often lead clinicians to the dictum of “start low, go slow” when administering new medications in older adults. However, for antibiotics, this is not an appropriate strategy. Altered gastric motility, decreased absorption, increased adipose tissue, and coadministration of other drugs can decrease blood levels of antimicrobials in the elderly patient, and since systemic antibiotics reach tissues via blood flow, poor vascular perfusion to the site of infection, particularly in skin and soft-tissue infections of the lower extremities, may reduce efficacy. Adherence may be limited by poor cognitive function, inadequate understanding of the drug regimen, impaired hearing or vision, and polypharmacy, and studies suggest that any regimen requiring greater than twice daily dosing is associated with very poor adherence rates. Finally, there are data that suggest higher antibiotic levels are particularly important for efficacy in older adults. This is true for antibiotics that are “concentration-dependent” (i.e., the higher the concentration in relation to the minimal inhibitory concentration (MIC), the better the antibiotic kills). The activity of fluoroquinolones is concentration-dependent and the relationship between bacterial clearance and the serum/MIC ratio for levofloxacin suggests drug concentrations must be nearly twice as high in older adults than in young adults to clear infection. The reason for this is unclear, but may be caused by impaired defense mechanisms described above that aid clearance of bacteria.
Many ethical dilemmas surround antibiotic use in frail elderly persons and terminally ill patients. The 1998 American Medical Association (AMA) Council of Ethical and Judicial Affairs included antibiotics, along with mechanical ventilation, as “life-sustaining” treatment. Others argue that antibiotics are part of ordinary care, even those who are designated to be receiving “comfort measures only.” While every clinical situation is unique, and no blanket recommendation can be made for the use or nonuse of antibiotics in the terminally ill, it seems prudent to include antibiotic administration in the discussion of advanced directives as a potentially life-sustaining maneuver and to treat it no differently than any other medical intervention such as surgery or mechanical ventilation.
Finally, although older adults are at the greatest risk for illnesses that are indications for outpatient parenteral antibiotic therapy (OPAT) (e.g., for treatment of endocarditis or osteomyelitis), OPAT was infrequently used in older adults primarily because of the fact that Medicare did not cover the cost of OPAT and older adults frequently had to be admitted to nursing homes or stay in acute care hospitals to obtain appropriate therapy. With the institution of Medicare Part D in 2006, OPAT is now covered, although navigating the system to obtain reimbursement for both the antibiotic and the necessary supplies for administration takes considerable skill. A recent study and accompanying editorial outline the safety, efficacy, and patient selection criteria for OPAT in older adults, and provides Web links to sites that assist with reimbursement issues (see Malani, 2008).
Clinical Syndromes in the Elderly
Although infective endocarditis (IE) was primarily a disease of young and middle-aged adults and associated with postrheumatic fever and congenital valvular lesions, it has become a disease of the elderly population, associated with degenerative valvular disorders and prosthetic valves (prosthetic valve endocarditis [PVE]). Furthermore, temporary and permanent pacemakers, pulmonary artery catheters, and other invasive devices are more frequently used in older adults and predispose subjects to IE.
Native valve IE in young adults is typically caused by viridans streptococci, S. aureus, and occasionally by HACEK organisms (Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, Kingella). These same organisms predominate in the elderly patient, but comorbidities and prosthetic devices change the profile of causative agents; gastrointestinal (GI) and genitourinary (GU) organisms such as enterococci and gram-negative rods become more common in native valve IE. Coagulase negative staphylococci are a frequent cause of PVE, both from contamination at the time of surgery and from occult or documented bacteremia during the hospital stay. Other nosocomial bacteremias, often with more resistant organisms (e.g., Enterobacter spp.), can also result in PVE.
Endocarditis is difficult to diagnose in the elderly patient. Fever and leukocytosis are less common (55% and 25%, respectively, for elderly patients vs. 80% and 60%, respectively, for younger patients). As stated above, the prominence of degenerative, calcific valvular lesions and prosthetic valves lowers the sensitivity of TTE. Transesophageal echocardiography (TEE) may be more sensitive, and in at least one study, TEE improved the diagnostic yield by 45% over that of TTE. Age alone is not a major risk for mortality, with a 2-year survival of 75% for IE in all age groups unless major comorbidities exist.
Antibiotic treatment of IE is directed at identified pathogens or the most likely causes if blood cultures are negative (Table 124-1). Therapy is administered intravenously for 2 to 6 weeks. Combination regimens with aminoglycosides are particularly problematic in the elderly patient because of toxicity (both renal and ototoxicity), but is occasionally unavoidable in certain circumstances (e.g., enterococcal IE). Surgical therapy is required only when specific criteria are met, primarily recurrent embolic events or worsening heart failure.
INFECTION | THERAPY | COMMENTS |
|---|---|---|
Community-acquired | ||
Outpatient Therapy | ||
Acute sinusitis | Amoxicillin | Amox/clav or new macrolide (clarithromycin or azithromycin) if refractory; new macrolide if penicillin allergic |
Chronic bronchitis | Amoxicillin-clavulanate | Infectious exacerbations only; new macrolide if penicillin allergic |
Pneumonia | [Azithromycin or Clarithromycin + 2nd gen Ceph or Amox/clav] or Resp-FQ | Smoker/COPD most common |
Cellulitis | Amox/clav or Cephalexin | Community-acquired MRSA much more common since 2005; include TMP-SMX or Minocycline if suspected (e.g., recurrent boils, very aggressive or abscess-forming skin/soft-tissue infection) |
Infected neuropathic ulcer | Amox/clav |