Trichosporon
T. asahii (T. beigelii)
T. inkin
T. mucoides Saccharomyces
S. cerevisiae
S. boulardii Rhodotorula
R. mucilaginosa (rubra)
R. glutinis Malassezia
M. furfur
M. pachydermati Blastoschizomyces capitatus (Geotrichum capitatum, Trichosporon capitatum) Sporobolomyces
S. salmonicolor
S. holsaticus
Trichosporon
Trichosporon asahii was first described in 1865 by Beigel, who identified it as the causative agent of hair infections [7]. Infections due to Trichosporon may be classified as superficial or deep. Disseminated infections are increasingly recognized in the compromised host over the past decade and are frequently fatal [1–6]. One of the first reported cases of disseminated disease was described in a 39 year-old female with lung cancer who subsequently developed a brain abscess [8].
Etiologic Agents
The genus Trichosporon was first reported by Behrend [9]. Gueho and colleagues have suggested that the species known as T. asahii may include several different Trichosporon species with epidemiological and pathogenic differences [10]. Kemker et al. using isoenzyme delineation and polymerase chain reaction (PCR) DNA fingerprinting suspect that strains that produce superficial infections are distinctly different from those strains that produce invasive infection [11]. There are currently seven species of Trichosporon. These include T. asahii (formerly, T. beigelii), the most frequently recovered species from invasive infections, and T. mucoides and T. inkin, also known to cause systemic infections [1, 2, 12–14]. T. asteroides and T. cutaneum generally produce superficial skin infections, while T. ovoides generally causes white piedra of the scalp and T. inkin, white piedra of the pubic hair. Trichosporon capitatum is now known as Blastoschizomyces capitatus [10, 12, 13].
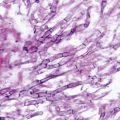
Trichosporon species are characterized by true hyphae, pseudohyphae, arthroconidia, and blastoconidia [10, 15] (Fig. 9.1). T. asahii grows readily on Sabouraud dextrose agar, producing smooth, shiny gray- to cream-colored yeast-like colonies with cerebriform radiating furrows that become dry and membranous with age. All Trichosporon species are easily identified using commercially available carbohydrate assimilation assays.
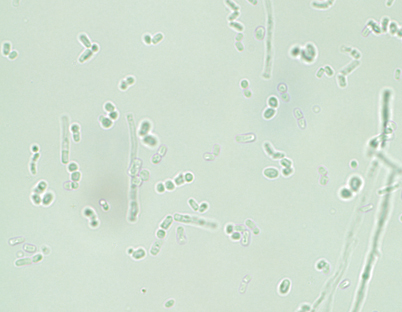

Fig. 9.1
Trichosporon species produce yeast-like colonies in culture and are unique in their production of hyphae, pseudohyphae, arthroconidia, and blastoconidia (budding) both in culture and in disease. (Courtesy of D. R. Hospenthal)
Epidemiology
T. asahii is generally found in the soil, but may also be recovered from air, rivers and lakes, sewage, and bird droppings [1, 2, 12]. It rarely colonizes the inanimate environment, but can colonize the mucosal surfaces of the oropharynx, the lower gastrointestinal (GI) tract, and the skin of humans [12, 13].
More than 100 documented cases of disseminated infection due to Trichosporon species have been reported, most due to T. asahii [1, 2, 12, 13, 15]. The major risk factors associated with infection include hematologic malignancies (acute leukemia, chronic leukemia, multiple myeloma), solid tumors, and neutropenia [1, 2, 12–15]. In non-neoplastic, non-neutropenic cases, the major risk factors include corticosteroids, prosthetic valve replacement, solid organ transplantation, chronic active hepatitis, and occasionally intravenous drug use (Table 9.2). The most common portal of entry appears to be either the respiratory or GI tracts. Infrequently, central venous catheters and other vascular devices have also been implicated [12–16].
Table 9.2
Risk factors associated with Trichosporon infection
• Hematologic malignancy |
• Solid organ transplantation (kidney, heart, liver) |
• Neutropenia |
• Broad-spectrum antibiotics |
• Corticosteroids |
• Use of intravenous lipids |
• Bone marrow transplant |
• Chronic active hepatitis |
• IVDU |
• Central venous catheters |
• CAPD |
• Burns |
Clinical Manifestations
Trichosporonosis is classified into superficial infections (white piedra (hair shaft infection), onychomycosis, and otomycosis) and invasive infections.
Deep tissue infections may involve either a single organ or multiple organs. The most commonly infected tissue is the lungs, which accounts for approximately 33 % of all localized deep tissue infections [1, 2, 12–19]. Other sites of infection may include the peritoneum, heart valves (natural and prosthetic), retina, liver, spleen, kidneys, gallbladder, and central nervous system (brain abscess and chronic fungal meningitis) [1,2, 10–19].
The signs and symptoms of disseminated infection resemble those of systemic candidiasis and include fungemia with associated organ infection. Moreover, disseminated infections may present as either acute or chronic disease. Acute disseminated trichosporonosis often has a sudden onset and progresses rapidly, especially in neutropenic patients. Patients may develop skin lesions (~ 33 %), pulmonary infiltrates (~ 30–60 %), renal, and ocular involvement.
The metastatic cutaneous lesions generally begin as an erythematous rash with raised papules on the trunk and the extremities. The rash eventually evolves into macronodular lesion, followed by central necrosis of the nodules and occasionally the formation of hemorrhagic bullae. The pulmonary infiltrates may present as lobar consolidations, bronchopneumonia, or reticulonodular patterns.
Renal involvement occurs in > 75 % of the disseminated infection cases. Renal disease may manifest as proteinuria, hematuria, red blood cell (RBC) casts, with either acute renal failure or acute glomerulonephritis [12–16]. Urine cultures are frequently positive for Trichosporon and suggest disseminated disease, especially in immunocompromised patients [19].
Chorioretinitis is not uncommon in disseminated infection and may be a cause of visual alterations due to retinal vein occlusion or retinal detachment [12–14]. For unexplained reasons, Trichosporon has been found to have tropism for the choroid and retina. However, unlike candidal endophthalmitis, Trichosporon infects uveal tissues including the iris, but spares the vitreous [20].
During disseminated infection, any tissue in the body may become infected. The organs most frequently include the liver, spleen, GI tract, lymph nodes, myocardium, bone marrow, pleura, brain, adrenal gland, and thyroid gland [1, 2, 12–20].
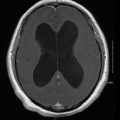
In chronic disseminated infection, subtle manifestations may be present for several weeks and frequently include persistent fever of unknown etiology [1, 2, 12, 13]. The infection is similar to the entity known as chronic disseminated (hepatosplenic) candidiasis. It is generally a chronic infection of the liver, spleen, and other tissues after recovery from neutropenia. Laboratory studies frequently reveal an elevated alkaline phosphatase. Computed tomography (CT) scan or magnetic resonance imaging (MRI) frequently reveals hepatic or splenic lesions compatible with abscesses. A tissue biopsy is needed to confirm the diagnosis.
Diagnosis
The diagnosis is made with a biopsy of the skin or involved organs. Blood cultures may occasionally be useful in deep tissue infection, but are positive only late in the course of infection. Trichosporon grows readily in conventional blood culture and on standard fungal media including Sabouraud dextrose agar [15]. The presence of Trichosporon in the urine of a high-risk patient should increase the suspicion of disseminated infection.
Although there are no standardized serologic assays, the serum latex agglutination test for C. neoformans may be positive. A potential usefulness of this assay has been postulated based on the report of positive serum latex agglutination test for C. neoformans in several patients with disseminated Trichosporon infection [21, 22].
Treatment
Disseminated trichosporonosis has a mortality rate of approximately 60–70 % [2–6, 12, 13]. In most cases, however, the underlying disease contributes greatly to the overall mortality. First-line, optimal antifungal therapy has not been established. The initial step in the management of disseminated Trichosporon infection should be to decrease or reverse immunosuppression.
In vitro susceptibility studies of Trichosporon species are limited (Table 9.3). In vitro susceptibility assays of T. asahii reveal fluconazole MIC90 of 4.0 µg/ml, itraconazole MIC90 of 0.25 µg/ml, and amphotericin B MIC90 of 4.0 µg/ml. In general, most strains have relatively high MICs for polyenes, flucytosine, and echinocandins, with relatively low MICs for the azoles. Among the newer triazoles, voriconazole and posaconazole have demonstrated excellent in vitro activity [22–26]. Although not yet approved, isavuconazole also shows significant in vitro activity against most strains of Trichosporon (Table 9.4) [25]. In fact, it appears to have better activity than fluconazole [25]. In vitro and animal models suggest that azoles and not polyenes are more effective in the eradication of Trichosporon species [1, 2, 20]. Suggested therapy for the treatment of disseminated disease includes the use of either voriconazole 3 mg/kg IV or 200 mg orally twice daily, fluconazole 400–800 mg/day, or itraconazole 400–600 mg/day (Table 9.4). A potential option in patients failing azole therapy may also include a combination of an azole with an echinocandin. Serena et al. demonstrated in vitro synergy and improved outcomes in an animal model of trichosporonosis with either the combination of amphotericin B/micafungin or fluconazole/micafungin [12, 14, 24, 25]. However, because of the high MICs to polyenes and echinocandins, these antifungals should not be used alone or as first-line therapy. In a patient with disseminated infection and poor response to therapy, in vitro susceptibility testing of recovered isolates may be a helpful adjunct.
Table 9.3
In vitro antifungal activity against emerging yeast infections
Minimum inhibitory concentration (MIC; µg/ml) rangea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Organism | Flu | Itra | Vori | Posa | Isuv | Mica | Cas | Anid | AMB | 5FC |
Trichosporon | 1–16 | 0.06–0.25 | 0.03– > 16 | 0.06- > 16 | 0.015–0.5 | 16–64 | 4– > 16 | 16– > 16 | 1–8 | 16– > 512 |
Saccharomyces | 0.5–64 | 0.03–4 | 0.016–2 | 0.12–1.0 | 0.03–1 | NA | 0.25–1 | 0.25–1 | 0.032–4 | < 0.125–1 |
Rhodotorula | 0.5– > 64 | 0.25- > 16 | 0.25– > 8 | 0.25– > 8.0 | 0.125–2 | > 64 | 8– > 64 | > 64 | 0.12–1.0 | 0.6–0.25 |
Malassezia | 1.0–16 | 0.03–0.25 | 0.03–0.125 | 0.03–32 | 0.03–0.5 | NA | NA | NA | 0.3–2.5 | > 100 |
Blastoschizomyces | 1–32 | 0.03–0.50 | 0.03–0.50 | 0.12–0.25 | NA | NA | NA | 1–4 | 0.6–0.25 | 0.12–16 |
Sporobolomyces | 1.25– > 64 | 1.0–2.0 | 0.25–4.0 | NA | NA | > 64 | NA | NA | 0.14–1.0 | NA |
Table 9.4
Suggested antifungal agents for use in the treatment of emerging yeast infections
Yeast | Antifungal therapya |
|---|---|
Trichosporon | Fluconazole 400 mg/day |
Voriconazole 200 mg bid | |
Itraconazole 400–600 mg/day | |
Saccharomyces | Amphotericin B |
5-Flucytosine | |
Azoles (ketoconazole, clotrimazole, miconazole) | |
Rhodotorula | Amphotericin B + 5-flucytosine |
Malassezia | Fluconazole 400 mg/day |
Voriconazole 200 mg bid | |
Amphotericin B 0.7 mg/kg/day | |
Blastoschizomyces | Amphotericin B 1–1.5 mg/kg/day |
Sporobolomyces | Amphotericin B |
Azoles (ketoconazole, itraconazole, fluconazole) |
Saccharomyces
Saccharomyces is an ascomycetous yeast found throughout nature. Saccharomyces is commonly known as “brewer’s yeast” or “baker’s yeast.” It is best known for its commercial use in beer and wine production, in health food supplements, and more recently, its use in DNA recombinant technology. Occasionally, these yeasts have been reported to cause severe infection in immunocompromised hosts [15]. Species include S. cerevisiae, S. boulardii (a subtype of S. cerevisiae), S. fragilis, and S. carlsbergensis. Saccharomyces may occasionally be part of the normal flora of the GI and genitourinary tracts [1, 2, 27]. Recently, S. cerevisiae has been found to cause mucosal and disseminated infection in humans, primarily in immunocompromised hosts [27–29].
Etiologic Agents
Cells are oval to spherical and exist as either haploids or diploids. When present, ascospores, one to four in number, are in either tetrahedral or linear arrangement and stain Gram negative; vegetative cells stain Gram positive. Colonies are smooth, moist, and either white or cream colored. Saccharomyces are generally nonpathogenic due to innate low virulence [15, 30–32]. Investigators evaluating more than 3300 yeast cultures obtained from cancer patients found only 19 isolates of S. cerevisiae. Recent studies by Clemons et al. using an animal model have been able to show that some strains of S. cerevisiae, when introduced into CD-1 mice, can proliferate and resist clearance in vivo, supporting the role of S. cerevisiae as a cause of clinical infection in humans [32].
Epidemiology
Isolation of Saccharomyces species from human surfaces is rarely of any clinical significance. It has been recovered from the bloodstream, lungs, peritoneal cavity, esophagus, urinary tract, and vagina [2–6, 27, 30, 33]. Genotyping studies evaluating the relatedness between clinical strains and commercial strains of S. cerevisiae have demonstrated that commercial products may occasionally be a contributing factor in human colonization and infection [34]. Nyirjesy et al. reported that four women suffering from recurrent S. cerevisiae vaginitis had also experienced exposure to bread dough that contained identical strains S. cerevisiae [29].
The risk factors associated with Saccharomyces infections are similar to the risk factors associated with candidemia and systemic candidiasis; including central venous catheters, neutropenia, use of antimicrobials, GI tract surgery, and occasionally HIV [28, 35–37]. The portal of entry for invasive disease is most likely the oropharynx or GI tract [36].
Clinical Manifestations
Manifestations are generally nonspecific and indistinguishable from those associated with candidemia and invasive candidiasis. In addition, Saccharomyces has been associated with bloodstream infections, endocarditis, peritonitis, disseminated disease, and vaginitis [1–6, 29, 33, 35–39].
Fungemia is the most common form of infection, occurring in approximately 70 % of reported cases. As in invasive candidiasis, it is seen primarily in the immunocompromised host and tends to be associated with the use of intravascular catheters, chemotherapy, and/or antimicrobials [27, 28, 36]. Manifestations are similar to those of systemic candidiasis and candidemia. Overall, fever unresponsive to broad-spectrum antimicrobials is the most frequent manifestation. Unlike infections due to Candida species, most patients survive.
In addition, it is not uncommon for other organ systems to become infected, including the respiratory tract, with several documented episodes of pneumonia and empyema. Diagnosis is generally established by histopathology, since Saccharomyces can colonize the respiratory tract without producing invasive disease [27, 36, 40]. Saccharomyces has also been reported to produce peritonitis, cholecystitis, and endocarditis [27, 36]. All cases of endocarditis were associated with prosthetic valves and intravenous heroin use. Furthermore, two out of the three patients were cured with antifungal therapy alone; only one patient had their valve replaced. There have also been several documented cases of urinary tract infections due to S. cerevisiae [29]. All patients had underlying urologic abnormalities or were associated with fungemia [36].
Mucosal infections due to S. cerevisiae have also been reported. Sobel et al. reported on 17 women with difficult to manage vaginitis due to S. cerevisiae [26, 29]. In fact, the women with symptomatic vaginitis had manifestations indistinguishable from those caused by C. albicans. All patients had a history of chronic vaginitis unresponsive to conventional antifungals and all but two had systemic or local predisposing factors.
Diagnosis
Because of the fact that Saccharomyces species have a tendency to be nonpathogenic, the decision to attribute a causal role to S. cerevisiae is difficult [2–6]. Diagnostic difficulty occurs when the organism is recovered from body sites that may be colonized by Saccharomyces, especially in the absence of symptoms of infection. Unless the organism is found in the bloodstream, it is frequently necessary to determine whether these yeasts are causing true infection versus colonization. This is generally done via a histopathologic examination. S. cerevisiae readily grows from blood culture bottles and on Sabouraud dextrose media [2–6, 34].
Treatment
It is often difficult to assess the role of antifungal therapy in patients with infection due to Saccharomyces. There are several reports that document the resolution of fungemia and infection just by removing the intravascular catheter without providing antifungal therapy [35, 36]. Most experts advocate removing the focus of infection, whether it is an indwelling or tunneled intravenous catheter and the concurrent use of antifungal agents [36]. In vitro susceptibility studies reveal that S. cerevisiae, when compared to C. albicans isolates, are less susceptible to most antifungals, including azoles [1–5, 26] (Table 9.3). Although clinical trials have not been conducted and in vitro susceptibility assays are not standardized, Saccharomyces species appear to be susceptible to most antifungals including amphotericin B, 5-flucytosine, ketoconazole, clotrimazole, miconazole, and terconazole [1–5, 26] (Table 9.4).
Rhodotorula
Yeasts of the genus Rhodotorula are found worldwide from a variety of sources and is generally considered a contaminant when identified. Infections are occasionally seen primarily in immunocompromised hosts [2–6].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree