Fig. 18.1
Number of PTx performed for sHPT in the 3rd General and Esophageal Surgical Unit, San Giovanni Battista Hospital, University of Turin, Italy from 1975 to 2014
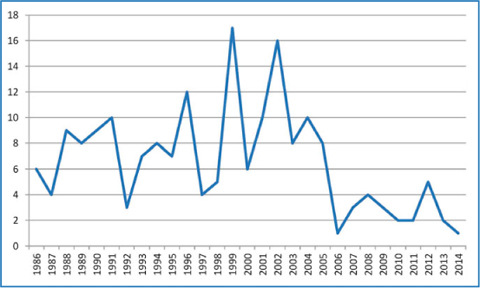
Fig. 18.2
Number of PTx performed for tHPT in 3rd General and Esophageal Surgical Unit, San Giovanni Battista Hospital, University of Turin, Italy from 1986 to 2014
Certainly, the duration of dialysis can influence the need for a PTx: the necessity of an intervention in patients treated with dialysis for more than 10 years was ten times higher than that in patients treated for less than 5 years. The incidence in patients in whom the disease appears or progresses after kidney transplantation, so-called tertiary HPT, is 6 to 7% [3].
Several factors are involved in the pathogenesis of refractory sHPT, particularly delayed and/or inadequate therapy, persistent hyperphosphatemia, an acquired increase in parathyroid gland mass due to polyclonal parathyroid cell proliferation (diffuse hyperplasia) and monoclonal expansion of adenomatous-like tissue (nodular hyperplasia) [4]. In fact a reduced renal mass causes a decreased serum 1,25-dihydroxyvitamin D3 and an increased serum phosphate. These two conditions are responsible for hypocalcemia and increased parathormone (PTH) secretion. A low serum 1,25-dihydroxyvitamin D3 is also the cause of a decreased number of vitamin D receptors, a decreased number of calciumsensing receptors, and the increase in serum phosphate, which directly act on the increase of PTH secretion [5].
18.2 Indications for Parathyroidectomy in Secondary Hyperparathyroidism
The new therapy for sHPT with oral calcitriol and vitamin D analogues, as well as calcimimetics, has lead to a decrease in PTx. However, it is very interesting to report the recent paper of Schneider and Bartsch; they claim that as much as 32% of patients require PTx after months or years of expensive medical treatment [2, 6]. The cost of calcimimetic therapy, such as cinacalcet, is about €240–1300 per month depending on the dose used. In contrast, the cost of a PTx is approximately €4000 and they conclude that it is potentially a definitive treatment; in any case, a successful kidney transplantation remains the optimal treatment for sHPT.
Following the suggestion of the 2003 Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guidelines [7] for bone metabolism and disease in chronic kidney disease, the indications for PTx in patients with chronic kidney disease (Guideline 14), are
severe hyperparathyroidism (persistent serum levels of intact PTH >800 pg/mL), associated with hypercalcemia and/or hyperphosphatemia, refractory to medical therapy
presence of calciphylaxis with PTH levels that are elevated (>500 pg/mL).
In 2009, the recommended target range for serum PTH in dialysis patients has changed from 150 to 300 pg/mL, in the KDOQI guidelines [7], to 2–9 times the upper limit of normal in the Kidney Disease Improving Global Outcomes (KDIGO) guidelines [8–9].
The problem concerns the interpretation of PTH in dialysis patients, but also the more global problem of establishing normal values for PTH in these patients, so you can assume that a PTH higher than 600 pg/mL could be dangerous [10].
The proposition of the KDIGO work group to use a target range for serum PTH based on the upper limit of the normal values is a pragmatic and elegant way to overcome variability in PTH assays [9]. In addition to these statements, it is necessary to stress that until now the indications for PTx have not been well defined and it is practically impossible to predict if medical therapy will be effective. In some cases ultrasonographic or radionuclide techniques could be useful as a predictor of efficacy of medical therapy considering the reduction of the parathyroid glands mass but there is insufficient evidence to support this at the present time.
The use of imaging procedures in sHPT and tHPT is questionable. Imaging of parathyroid glands with 99mTc-sestamibi (MIBI) scan, ultrasound, CT scan, or MRI should be done to identify ectopic glands or prior to re-exploration parathyroid surgery. The parathyroid weight correlates to MIBI scintigraphy uptake in both sHPT and tHPT. A significant difference between the weight of high-uptake parathyroids and low-uptake or no-uptake glands exists, but there is no difference between histology (diffuse or nodular hyperplasia) and MIBI scintigraphy of uptake and no-uptake glands. So MIBI scintigraphy does not help the surgeon identify the gland to be left in situ or tranplanted or cryopreserved [11, 12].
These results are in agreement with the findings in a number of studies concerning the utility of MIBI scintigraphy for sHPT and tHPT, in correlation with the weight, the cellular function, and the localization [13–17]. Others, however, do not recommend using MIBI scintigraphy, except for reoperations [12, 18–20].
Regarding the intraoperative parathyroid hormone (ioPTH) assay, we can consider our personal experience: for the 464 patients with sHPT and tHPT operated on from 1995 to 2007 for which we had a complete follow-up, 187 patients were operated on without using either preoperative MIBI scintigraphy or ioPTH assay, 209 were operated and had an ioPTH assay, and 68 had both. For patients with sHPT, when neither MIBI nor ioPTH were used, there was a persistence rate of 6.2% and a recurrence rate of 11%; in patients having an ioPTH assay without MIBI, both the persistence rate and the recurrence rate were 4.9%; in 48 patients having both, MIBI and ioPTH, there was one persistence and recurrence was 4.1%. Analyzing persistence and recurrence, we found a difference only between patients having neither ioPTH nor MIBI and those having both tests. We obtained similar results for patients operated on for tHPT. Analyzing recurrence, we found a difference between patients having no tests and those having ioPTH alone or both ioPTH and MIBI, but no difference between ioPTH alone, and ioPTH and MIBI. We had a successful outcome when removing only three glands without persistence or recurrence and this result could be due to asymmetric hyperplasia or even a fourth normal parathyroid glands in tHPT (the ioPTH with no false positive confirmed this statement).
The combination of ioPTH assay and preoperative MIBI scintigraphy appears to be helpful for the surgeon and decreases the risk of persistent HPT, but there is no statistically significant benefit in comparison with the use of the ioPTH assay alone.
A cut-off of 81–82% below the initial PTH value for sHPT was used as can be seen in Table 18.1. Also considering the different curve of PTH in these patients, it offers a highly predictive evaluation of the persistence of the disease. In tHPT as in reoperations the cut-off could be 50%, except in difficult cases when it should be 68% [11]. Normally two samples were taken, the first immediately before the anesthesia and the second one 10 minutes after Subscripttotal resection or removal of all the glands with autotransplantation. If there was some uncertainty, we obtained a third sample 20 minutes after PTx [11, 21–24]. In our opinion, the quick ioPTH is useful not only in primary HPT, but also in sHPT and tHPT, because it improves short term results and predicts a persistence; it is absolutely essential for reoperations. Certainly, other factors must be taken into consideration in long-term follow-up.
Table 18.1
Quick intraoperative PTH (ioPTH) usefulness in secondary hyperparathyroidism (sHPT) and tertiary hyperparathyroidism (tHPT)*
Sensitivity | Specificity | TP | TN | FP | FN | |||
|---|---|---|---|---|---|---|---|---|
% | % | |||||||
sHPT | t0-t10 | Cut-off 50% | 96.2 | 66.7 | 151 | 4 | 2 | 6 |
Cut-off 75% | 68.8 | 83.3 | 108 | 5 | 1 | 49 | ||
Cut-off 81% | 42.0 | 100.0 | 66 | 6 | 0 | 91 | ||
More samples | Cut-off 50% | 99.4 | 50.0 | 156 | 3 | 3 | 1 | |
Cut-off 75% | 77.1 | 83.3 | 121 | 5 | 1 | 36 | ||
Cut-off 82% | 43.9 | 100.0 | 69 | 6 | 0 | 88 | ||
tHPT | t0-t10 | Cut-off 50% | 85.0 | 100.0 | 34 | 2 | 0 | 6 |
Cut-off 68% | 70.0 | 100.0 | 28 | 2 | 0 | 12 | ||
More samples | Cut-off 50% | 90.0 | 50.0 | 37 | 1 | 1 | 3 | |
Cut-off 68% | 83.0 | 100.0 | 33 | 2 | 0 | 7 |
It is our opinion that it is mandatory to keep in mind the recent findings of the Mount Sinay Hospital regarding the cost of the method, but also remembering the reported aphorism of Doctor Jensen: “surgeon who strives for perfection needs some basis for patient selection. He would like to be sure there’s a good chance for cure before he begins [ends in our cases] the resection”. In conclusion, the indication for surgery is often a failure of the pharmacologic therapy due to poor patient compliance during dialysis, with worsening of uremic osteodystrophy. Subscriptperiosteal lesions of the hands and feet phalanges, acromioclavicular fractures, pubic symphysis diastasis, salt-and-pepper skull lesions, or vascular and ectopic calcifications and pruritus are diagnostic. The most important biochemical findings include elevated levels of intact parathyroid hormone >800 pg/mL in association with symptomatology. The PTH level at which PTx is indicated for asymptomatic patients is not known. Some authors commonly refer for PTx when the patient has a PTH level >1000 pg/mL, but the 2009 KDIGO guidelines do not specify a PTH level that is an indication for PTx [8, 25, 26].
The degree of hypercalcemia is not so important: it becomes relevant only when its concentration exceeds 10.5 mg/dL, indicating autonomous function of one or more glands. Obviously, patients with a low level of calcium must be treated with supplementary calcium and vitamin D, but in many cases the level of parathyroid hormone remains high. If the product of calcium and phosphate is greater than 70, this is another main indication for PTx to prevent vascular and ectopic calcifications.
A more recent interpretation of the clinical aspect of these patients in the calcimimetic era suggests the following indications for PTx [27]:
average iPTH >800 pg/ml despite optimal available therapy
average iPTH >500 pg/ml despite optimal available therapy in addition to:
serum calcium >9.5 mg/dl or
serum phosphate >5.5 mg/dl or
Ca x P product >55 or
progressive loss of hip or lumbar spine bone mineral density in patients with osteoporosis on optimal therapy.
A controversial issue is whether patients with markedly elevated, refractory PTH levels without signs or symptoms should also undergo PTx. The situation is more complicated for patients awaiting a kidney transplant: they should undergo surgical treatment, without any hesitation, before kidney transplantation, if the intact parathyroid hormone level exceeds 800 pg/mL, despite adequate medical therapy, to avoid injury to the graft [11, 28].
18.3 Indications for Parathyroidectomy After Renal Transplantation
HPT persists after transplantation in about 40% of patients, even when the kidney function is good. Approximately 3–5% of patients require PTx after transplantation, and this is called tHPT or autonomous HPT. The indication for PTx in these cases has to be carefully considered because there is often a decrease in renal function or even transplant failure after the operation. If hyperparathyroidism is not corrected, other lesions may appear that are related to the immunosuppressive and corticoid therapy. These include rupture of ligaments and tendons, metastatic calcifications, and calciphylaxis [11, 28, 29]. In these cases, the concentration of calcium is expected to be high. A high S-phase fraction and several histologic parameters such as total parenchymal mass, nodular growth pattern, absence of fat cells, and high mitotic index have been proposed as markers of poor parathyroid suppressibility [1, 30–33]. In a recent study of parathyroid tumors (adenomas and carcinomas), we proposed that high proliferative activity, as detected by Ki-67 antigen expression, is an adjunct to the histopathologic diagnosis of parathyroid lesions that can be used to discriminate clinically aggressive from clinically indolent cases [34]. In conclusion, hypercalcemia and an elevated PTH, 6 months after transplantation, are clearly indications for PTx.
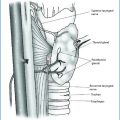
18.4 Surgical Approaches
Before surgery, it is absolutely necessary to accurately prepare the patient with two sessions of dialysis just before the operation so as to have the potassium within the normal range and to avoid untoward incidents during anesthesia, due to hyperkalemia, or the necessity to undergo dialysis immediately after surgery.
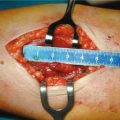
Effective surgical therapy of severe sHPT can be accomplished by Subscripttotal PTx, or by total PTx with parathyroid tissue autotransplantation. If you perform total PTx with autotransplantation we recommend cryopreservation. In our opinion, Subscripttotal PTx could be the treatment of choice because of the shorter surgical time, and avoiding the need for cryopreservation. Total PTx with auto-transplantation and cryopreservation is indicated when the glands are in an ectopic position or difficult to save due to their poor vascularization or if they are very big and difficult to cut. This operation is also indicated in the case of persistence or recurrence. In our opinion, total PTx is not indicated except in patients older than 70 who definitely do not have the possibility of transplantation because of postoperative hypoparathyroidism with all the consequences connected to this disease.
This restricts the use of total PTx to a few selected older patients, who are not candidates for renal transplantation.
The surgical technique is similar to the one utilized for hyperplasia in primary HPT. In recent years if patients treated with calcimimetics are operated, it is possible that some glands regress and others do not, so sometimes it is difficult to identify normal glands for a so-called asymmetric hyperplasia. In our experience, all surgical procedures caused regression of osteodystrophy, pruritus, and ectopic calcifications but not vascular calcifications.
The percentage of recurrence in our experience was 5 to 8% and did not differ among the various types of procedures. However, after total PTx, many patients had permanent hypocalcemia. Less satisfactory results were obtained after incomplete PTx; when a gland was not found, the recurrence rate was 34%. This percentage does not include recurrence resulting from a fifth parathyroid gland, which was present in 32 patients.
When we examined the patients who underwent surgery for tHPT (hyperparathyroidism that develops after renal transplantation), we noted that the regression of osteodystrophy and ectopic calcifications was satisfactory after either Subscripttotal or total PTx and autotransplantation, with no recurrence and no hypocalcemia.
In the reoperative setting, total PTx could be an alternative option to total PTx with autotransplantation, but always with cryopreservation: it avoids the possibility of a third cervicotomy and autotransplantation could be performed later in the case of hypocalcemia. We performed autotransplantation after 3 months in three patients with satisfactory results. In one patient it was performed 1 year after cryopreservation: histologic specimens were normal, but the transplant did not show any hormonal activity. In conclusion, the cryopreserved tissue may not function as well as primary autotransplanted parathyroid tissue, particularly after months of cryopreservation, so we do not suggest waiting more than 3 to 6 months after the previous operation.
In patients who underwent renal transplantation early after PTx and only three glands were removed, recurrence was reduced to 20%. Therefore, kidney transplantation is the principal objective after PTx. Patients that are high risk for recurrence should be referred for early renal transplantation as soon as possible after PTx [35].
The choice of the gland to be left in situ or to be transplanted is very important. From a macroscopic standpoint, the gland left in situ or transplanted should always be the smallest one and should have fatty tissue surrounding it. The histologic characteristics of a portion of the gland left in situ or transplanted were studied morphologically and immunohistochemically. In the absence of definitive criteria to identify patients at risk of recurrence, we believe that some morphologic aspects (primarily diffuse vs. nodular enlargement, but also proliferative cellular activity) can be used to distinguish patients at increased risk. In the literature, the nodular form rather than the diffuse form is more frequently responsible for recurrence [32, 36, 37]. This is confirmed by our observations that the nodular form was present in 95% of recurrences and 74% of controls. The mean proliferative index as determined by the Ki-67 technique was significantly greater (1.9%) in the recurrent group than in the controls (0.81%). In terms of the histologic characteristic of cells, the clear cells had a proliferative index activity greater than that of the chief cells. Obviously, a missed supernumerary parathyroid gland could contribute to surgical failures: sometimes in these cases, glands are only remnants and after a period of good recovery the recurrence appears.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree