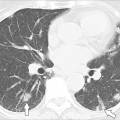
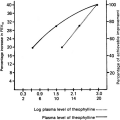
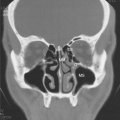
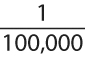Immunology of IgE-mediated and Other Hypersensitivity Responses
C. Raymond Zeiss
Historical Review of IgE-Mediated Hypersensitivity
In 1902, Richet and Portier described the development of anaphylaxis in dogs given sea anemone toxin; subsequently, anaphylaxis was described in humans after the injection of horse serum to achieve passive immunization against tetanus and diphtheria. In 1906, Clemens von Pirquet correctly predicted that immunity and hypersensitivity reactions would depend on the interaction between a foreign substance and the immune system, and that immunity and hypersensitivity would have similar underlying immunologic mechanisms (1).
The search for the factor responsible for immediate hypersensitivity reactions became a subject of intense investigation over several years. The transfer of allergy to horse dander by transfusion was reported by Ramirez in 1919 (2). In 1921, Prausnitz and Küstner (3) described the transfer of immediate hypersensitivity (to fish protein) by serum to the skin of a normal individual. This test for the serum factor responsible for immediate hypersensitivity reactions was termed the Prausnitz-Küstner test. Variations of this test remained the standard for measuring skin sensitizing antibody over the next 50 years.
In 1925, Coca and Grove (4) extensively studied the skin-sensitizing factor from sera of patients with ragweed hay fever. They called skin-sensitizing antibody atopic reagin because of its association with hereditary conditions and because of their uncertainty as to the nature of the antibody involved. Thereafter, this factor was called atopic reagin, reaginic antibody, or skin-sensitizing antibody. This antibody clearly had unusual properties and could not be measured readily by standard immunologic methods. Major research efforts from the 1920s through the 1960s defined its physical and chemical properties and measured its presence in allergic individuals (5,6).
In 1967, the Ishizakas discovered that skin-sensitizing antibody belonged to a unique class of immunoglobulin, which they called immunoglobulin E (IgE). In elegant studies using immunologic techniques, they clearly demonstrated that reagin-rich serum fractions from a patient with ragweed hay fever belonged to a unique class of immunoglobulin (7). Shortly thereafter, the Swedish researchers Johansson and Bennich discovered a new myeloma protein, termed IgND, which had no antigenic relation to the other immunoglobulin classes. In 1969, cooperative studies between these workers and Ishizakas confirmed that the proteins were identical and that a new class of immunoglobulin, IgE, had been discovered (8,9).
Physiology of IgE
IgE Structure and Receptors
The immunochemical properties of IgE are shown in Table 2.1 in contrast to those of the other immunoglobulin classes. IgE is a glycoprotein that has a molecular weight of 190,000 with a sedimentation coefficient of 8S. Like all immunoglobulins, IgE has a four-chain structure with two light chains and two heavy chains. The heavy chains contain five domains (one variable and four constant regions) that carry unique, antigenic specificities termed the epsilon (ε) determinants (Fig. 2.1A). These unique antigenic structures determine the class specificity of this protein. Digestion with papain yields the Fc fragment, which contains the epsilon antigenic determinants and two Fab fragments. The Fab fragments contain the antigen-combining sites. The tertiary structure of the Fc fragment is responsible for the protein’s ability to bind to the FcεRI receptors on mast cells and basophils (10).
Table 2.1 Immunoglobulin Isotypes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The FcεR1 receptor is the high-affinity receptor for IgE found on mast cells, basophils, eosinophils, and human skin Langerhans cells (11). Cross-linking of high-affinity receptor-bound IgE by allergen results in the release of mediators from mast cells and basophils. Molecular biologic techniques have been used to clone the gene encoding the e chain of human IgE (ND) and
to determine the site on IgE that binds to its receptor (12). Studies have localized this site to the Cε3 heavy chain domains (13). The high-affinity receptor for IgE is composed of an α chain, a β chain, and two γ chains, and it is the α chain that binds IgE (Fig. 2.1B). The crystal structure of the α chain has been determined, giving insights into the interaction of IgE with its receptor at the molecular level (14,15). The β and γ chains are involved in signal transduction when the receptors are aggregated by the cross-linking of IgE, resulting in mediator release (16). The IgE receptor on dendritic and other antigen-presenting cells is expressed in a heterotrimeric form α,γ,γ and is called FcεRII or CD23 (17,18). The capture of IgE allergen complexes by the dendritic cell IgE receptor is a highly efficient mechanism for allergen presentation to T cells (17).
to determine the site on IgE that binds to its receptor (12). Studies have localized this site to the Cε3 heavy chain domains (13). The high-affinity receptor for IgE is composed of an α chain, a β chain, and two γ chains, and it is the α chain that binds IgE (Fig. 2.1B). The crystal structure of the α chain has been determined, giving insights into the interaction of IgE with its receptor at the molecular level (14,15). The β and γ chains are involved in signal transduction when the receptors are aggregated by the cross-linking of IgE, resulting in mediator release (16). The IgE receptor on dendritic and other antigen-presenting cells is expressed in a heterotrimeric form α,γ,γ and is called FcεRII or CD23 (17,18). The capture of IgE allergen complexes by the dendritic cell IgE receptor is a highly efficient mechanism for allergen presentation to T cells (17).
Studies have delineated the central role that IgE molecules in the circulation play in determining the number of FcεRI receptors on mast cells and basophils (19,20) and consequently the release of mediators from these cells. After infusion of anti-IgE monoclonal antibody in allergic subjects, there is a significant reduction in serum levels of free IgE, with a dramatic fall in basophil FcεRI number and mediator release; of note, the total serum IgE is not reduced. The availability of anti-IgE monoclonal antibodies for the treatment of allergic
subjects has led to a wealth of information on the complex physiology of lowering free IgE levels in serum (17). The monoclonal anti-IgE antibody, omalizumab, binds circulating IgE at the same site in the Cε3 domain as the FcεRI receptor. It therefore binds free IgE and not FcεRI receptor-bound IgE on mast cells and basophils, which would lead to mediator release (17). A low-affinity FcεRII receptor (CD23) has been localized to B lymphocytes, monocytes, macrophages, platelets, eosinophils, and epithelial cells (15, 21). The receptor has an A form found only on B lymphocytes and a B form found on all cells expressing CD23. The molecular structure of CD23 has been delineated in detail; its binding site for IgE has three domains and in this trimeric form has a binding affinity for the IgE Cε3 region approaching that of the FceR1 receptor (15). The expression of this receptor is markedly up-regulated on all cell types by interleukin-4 (IL-4) and IL-13. Binding of IgE to this receptor places IgE at the center of activation of many important effector cells and adds an additional receptor on the B cell whereby IgE can present allergen to T cells (15,21). The role of CD23 in regulation of the IgE response is complex, having both positive and inhibitory effects (15,21).
subjects has led to a wealth of information on the complex physiology of lowering free IgE levels in serum (17). The monoclonal anti-IgE antibody, omalizumab, binds circulating IgE at the same site in the Cε3 domain as the FcεRI receptor. It therefore binds free IgE and not FcεRI receptor-bound IgE on mast cells and basophils, which would lead to mediator release (17). A low-affinity FcεRII receptor (CD23) has been localized to B lymphocytes, monocytes, macrophages, platelets, eosinophils, and epithelial cells (15, 21). The receptor has an A form found only on B lymphocytes and a B form found on all cells expressing CD23. The molecular structure of CD23 has been delineated in detail; its binding site for IgE has three domains and in this trimeric form has a binding affinity for the IgE Cε3 region approaching that of the FceR1 receptor (15). The expression of this receptor is markedly up-regulated on all cell types by interleukin-4 (IL-4) and IL-13. Binding of IgE to this receptor places IgE at the center of activation of many important effector cells and adds an additional receptor on the B cell whereby IgE can present allergen to T cells (15,21). The role of CD23 in regulation of the IgE response is complex, having both positive and inhibitory effects (15,21).
Sites of IgE Production, Turnover, and Tissue Localization
With the advent of a highly specific reagent for detecting IgE antibody against the Fc portion of IgE (anti-IgE), the sites of production of this immunoglobulin could be examined by fluorescent-labeled anti-IgE. It was found that lymphoid tissue of the tonsils, adenoids, and the bronchial and peritoneal areas contained IgE-forming plasma cells. IgE-forming plasma cells also were found in the respiratory and intestinal mucosa (22). This distribution is similar to that of IgA. However, unlike IgA, IgE is not associated with a secretory piece, although IgE is found in respiratory and intestinal secretions. The traffic of IgE molecules from areas of production to the tissues and the circulation has not been established. Areas of production in the respiratory and intestinal mucosa are associated with the presence of tissue mast cells (23). There has been renewed interest in the local mucosal production of IgE by IgE+ B cells. In grass- and ragweed-sensitive individuals there is clear evidence of marked local production of specific IgE after allergen challenge using elegant techniques to track gene activation (class switch recombination) involved in IgE production by B cells (24). It is speculated that most IgE production occurs in mucosal tissue sites (15,24)
With the development of techniques to measure total IgE in the blood and the availability of purified IgE protein, investigators were able to study the metabolic properties of this immunoglobulin in normal individuals (25). The mean total circulating IgE pool was found to be 3.3 μg/kg of body weight, in contrast to the total circulating IgG pool of about 500,000 μg/kg of body weight. IgE has an intravascular half-life of only 2.3 days. The rate of IgE production was found to be 2.3 μg/kg/day.
It had been known for several years that the half-life of reaginic antibody in human skin as determined by passive transfer studies was about 14 days. This was reconfirmed with studies that investigated the disappearance of radiolabeled IgE in human skin. The half-life in the skin was reported to be between 8 days and 14 days (7). The basophil and mast cell-bound IgE pool needs to be investigated thoroughly, but it has been estimated that only 1% of the total IgE is cell-bound. Direct quantification of specific IgE in the blood, in contrast to specific IgE on the basophil surface, indicates that for every IgE molecule on the basophil, there are 100 to 4,000 molecules in circulation (26).