Figure 115.1 Recommended immunization schedule for persons 0 through 18 years – United States – 2014. Source: http://cdc.gov/vaccines/schedules. Accessed December 7, 2014.
In the following summary, minimal ages for administration of the first dose of the various vaccines are given, and the detailed schedule for subsequent doses may be found in Figure 115.1. Figure 115.2 provides the CDC ACIP schedule for catch-up immunizations. The minimal interval between doses shown in this figure can be used to reset the schedule for a given vaccine series in persons aged 4 months through 18 years who start late or who are more than 1 month behind the recommended schedule. If more time elapses than the recommended interval between scheduled doses in a vaccine series, the series does not need to be restarted, but the next dose(s) should be administered according to the recommended intervals until the series is complete.
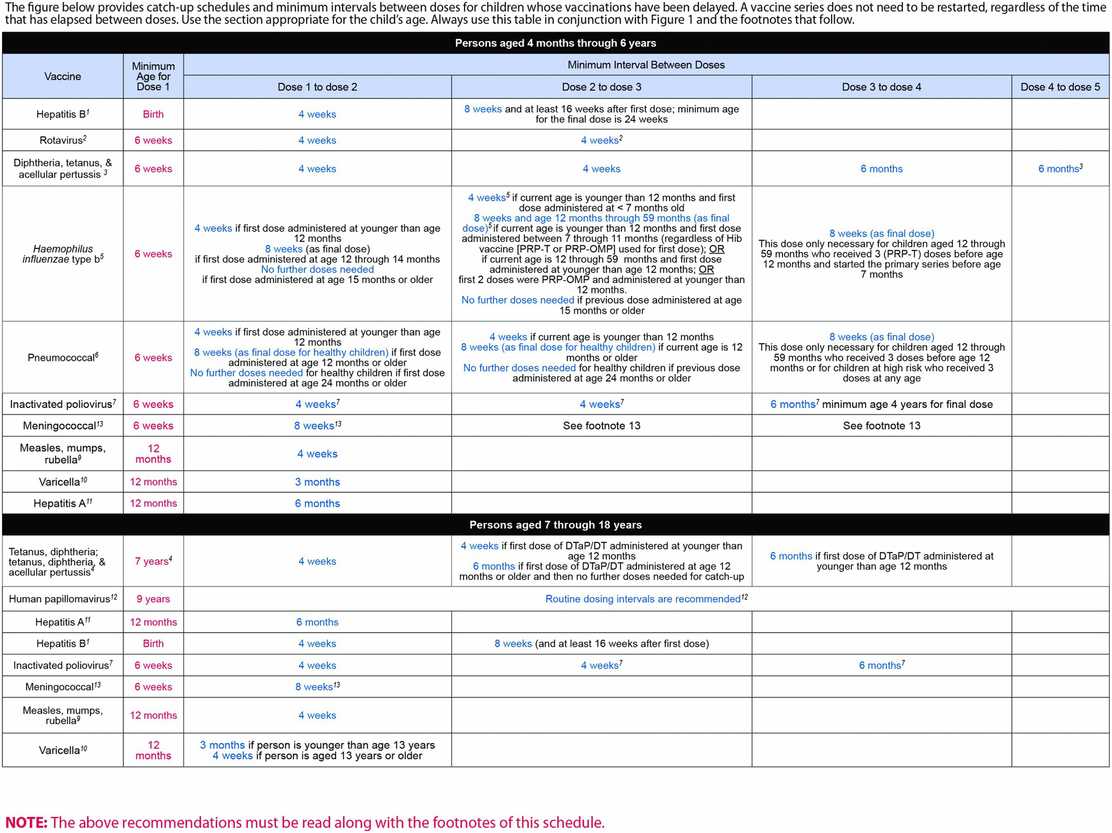



Figure 115.2 Catch-up immunization schedule for persons aged 4 months through 18 years – United States – 2014. Source: http://cdc.gov/vaccine/schedules. Accessed December 7, 2014.
At birth, hepatitis B (HepB: Recombivax HB, Merck; Engerix-B, GlaxoSmithKline) vaccination is initiated, and at 6 weeks of age, rotavirus (RV-1: Rotarix, GlaxoSmithKline; RV-5: RotaTeq, Merck), diphtheria and tetanus toxoids and acellular pertussis (DTaP: Daptacel, sanofi pasteur; Infanrix, GlaxoSmithKline), Haemophilus influenzae type b conjugate (Hib: HibTITER, Wyeth; PedvaxHIB, Merck; ActHIB, sanofi pasteur), pneumococcal conjugate (PCV13: Prevnar 13, sanofi pasteur) and inactivated poliovirus (IPV: Ipol, sanofi pasteur) vaccines are administered. At 6 months of age, annual vaccination with inactivated influenza vaccine (IIV: Fluarix, GlaxoSmithKline; Fluvirin, Chiron; Fluzone, sanofi pasteur) commences, and at 12 months of age, the first dose of measles, mumps, and rubella (MMR: M-M-R II, Merck), varicella (VAR: Varivax, Merck) and hepatitis A (HepA: Havrix, GlaxoSmithKline; Vaqta, Merck) vaccines are recommended. Varicella vaccine may be administered at the same time as the MMR vaccine. Both VAR and MMR are live-virus vaccines administered by injection. If the two vaccines are not given simultaneously at different sites, the vaccine doses should be separated by 28 days if possible to reduce or eliminate possible interference of the vaccine given first with the vaccine given second. A second dose of VAR is recommended for children, between 4 and 6 years old, on entry into school to boost waning immunity from the first dose received in infancy. At 2 years of age and older, annual vaccination against influenza may be done with either the inactivated influenza vaccine (IIV) given by injection, or the live attenuated influenza vaccine (LAIV: FluMist, MedImmune) administered intranasally.
The ACIP recommends vaccination of adolescents at 11 to 12 years of age with quadrivalent meningococcal conjugate vaccine (MCV4) with a booster at 16 years old. Until recently, two meningococcal conjugate vaccines are licensed: meningococcal (Groups A, C, Y-W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV4-D: Menactra, sanofi pasteur); and meningococcal (Groups A, C, Y, W-135) polysaccharide CRM197 conjugate vaccine (MCV4-CRM: Menveo, Novartis). If the first dose of MCV4-D or MCV4-CRM is received between 13 and 15 years old, a booster should be given at 18 years of age. Recently, a serogroup B vaccine has become available (see p. 940 for more details).
The immunization status of tetanus, diphtheria, and acellular pertussis vaccine should be reviewed among 11- to 12-year-olds as well. The ACIP recommends a single dose of the tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine for persons aged 11 through 18 years who completed the recommended childhood DTP/DTaP vaccinations series. There are two Tdap vaccines: one is licensed for use in persons aged 10 through 64 years (Boostrix, GlaxoSmithKline) and the other is licensed for use in persons aged 11 through 64 years (Adacel, sanofi pasteur), and both are licensed for use at an interval of at least 5 years between the Td and Tdap dose. However, due to the poor control of pertussis observed in the United States in recent years, ACIP now recommends expanded use of Tdap. Tdap vaccine may be given regardless of interval since the last tetanus-toxoid- or diphtheria-toxoid-containing vaccine, it may be used in undervaccinated children aged 7 through 10 years, and it may be used in certain adults aged 65 years and older according to current ACIP recommendations.
The human papillomavirus vaccine (HPV) should also be considered at the pre-adolescent health visit at 11 to 12 years old according to ACIP recommendations. There are two licensed HPV vaccines, one bivalent (HPV2) and the other quadrivalent (HPV4). HPV4 (Gardisil, Merck) vaccine is directed against two oncogenic types (HPV 16 and 18) and two nononcogenic types (HPV 6 and 11) associated with genital warts. HPV4 was originally licensed in 2006 for use in females 9 to 26 years old. HPV2 (Cervarix, GlaxoSmithKline) directed against the two oncogenic types (HPV 16 and 18) was licensed in 2009 for use in females 10 to 25 years old. Both HPV vaccines consist of a series of three doses given by intramuscular injection. The vaccines are composed of virus-like particles (VLPs) prepared from recombinant L1 capsid protein of human papillomavirus together with an adjuvant, and are not live vaccines.
ACIP recommends routine HPV vaccination of females aged 11 or 12 years old, catch-up vaccination for females aged 13 to 26 years old, and administration as early as 9 years old if the girl is at risk of exposure. HPV vaccine is contraindicated in pregnancy, but may be used in lactating women.
In 2009, the FDA expanded licensure for use of HPV4 in males 9 to 26 years old based on risk. In 2011, ACIP issued recommendations for routine HPV4 vaccination of males aged 11 to 12 years, and males 13 to 21 years not vaccinated previously. HPV4 vaccination may decrease the likelihood of genital warts. Additionally, the ACIP recommends routine HPV4 vaccination through the age of 26 years for men who have sex with men (MSM) because of increased risk for disease associated with HPV types 6, 11, 16, and 18, and for immunocompromised males (including those with HIV infection).
Although clinical trials of concomitant administration of HPV vaccine with the other vaccines recommended for adolescents have not been performed, there are no theoretical reasons not to administer the first dose of HPV during the same clinic visit as MCV4 and Tdap vaccines, with the vaccines being administered at different anatomic sites.
Combination vaccines
The number of recommended early childhood immunizations creates issues of compliance and scheduling for parents, patients, and healthcare providers. Depending on the availability and use of existing combination vaccines and new vaccines presently under development, the number of immunization injections per clinic visit can be decreased. Several commercially prepared combination vaccines are available for pediatric use (Table 115.1) and their use is encouraged to promote patient (parent) compliance. However, additional care must be taken in terms of scheduling missed doses, completing a vaccine series with a different vaccine product than originally used, and recognizing differences in antigen content and dose scheduling between monovalent vaccines and corresponding combination vaccines.
| Vaccine product | Age range |
|---|---|
| DTaP-IPV-HepB (Pediarix®, GlaxoSmithKline) | 6 weeks through 7 years |
| DTap-IPV/Hib (Pentacel®, sanofi pasteur) | 2 months through 5 years |
| DTaP-IPV (Kinrix®, GlaxoSmithKline) | 4 through 6 years |
| HepA-HepB (Twinrix®, GlaxoSmithKline) | 18 years or older |
| Hib-HepB (Comvax®, Merck) | 6 weeks through 5 years |
| MMR-Var (ProQuad®, Merck) | 12 months through 12 years |
a Consult the package insert for details of licensed use for each combination vaccine.
Adult immunizations
Recommendations for adult immunizations are based on the history of immunizations received in the past and on the need to give booster doses for certain vaccines. A detailed review of the personal immunization history is indicated for international travelers, healthcare workers, and other persons who have risks of exposure related to occupational activities, advanced age, or compromised immune status due to disease (HIV), medications, cancer, or other chronic medical conditions.
The immunization history should be updated and documented at the time of initial intake into a primary care practice, during interim health maintenance visits, on employment in one of the healthcare or social services professions, and/or prior to international travel. Travel immunizations will be covered in Chapter 116, Advice for travelers. If the immunization history of the person is uncertain or unknown, a conceptual framework of the prevalent practices pertaining to childhood, school, military service, and occupational immunization programs and standards will be helpful for assessing the current immunization status. The recommended adult immunization schedule is given in Figure 115.3.
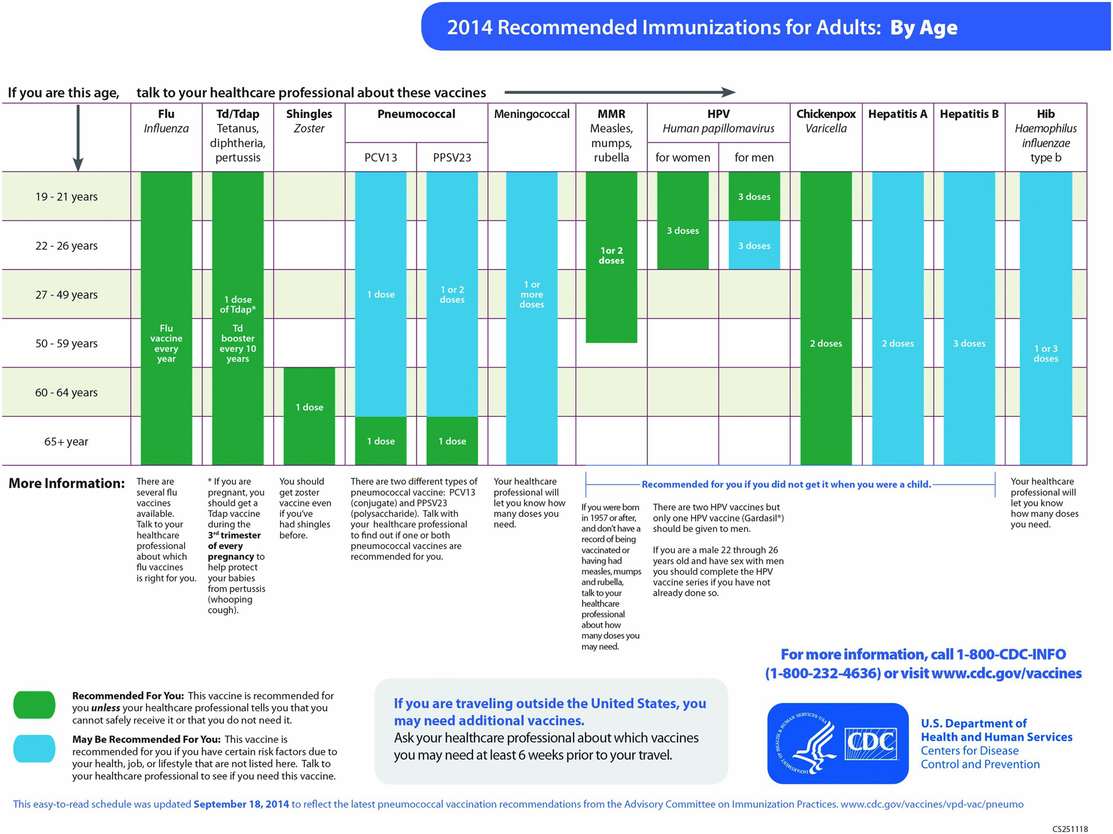
Figure 115.3 Recommended adult immunization schedule by age – United States – 2014. Source: http://cdc.gov/vaccines/schedules. Accessed December 7, 2014.
Routine immunizations for adults
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree