Immune thrombocytopenia (ITP) is a common hematologic disorder characterized by isolated thrombocytopenia. ITP presents as a primary or a secondary form. ITP may affect individuals of all ages, with peaks during childhood and in the elderly, in whom the age-specific incidence of ITP is greatest. Bleeding is the most common clinical manifestation of ITP. The pathogenesis of ITP is complex, involving alterations in humoral and cellular immunity. Corticosteroids remain the most common first line therapy for ITP. This article summarizes the classification and diagnosis of primary and secondary ITP, as well as the pathogenesis and options for treatment.
Key points
- •
Primary immune thrombocytopenia (ITP) presents as isolated thrombocytopenia (platelet count <100 × 10 9 /L) in the absence of other causes or disorders that may be associated with thrombocytopenia, or as a secondary disorder, most commonly associated with autoimmune disease (such as systemic lupus erythematosus) or chronic infections (such as Helicobacter pylori or hepatitis C).
- •
Primary ITP in children is usually self-limited, with approximately 80% of cases resolving within 6 to 12 months. In contrast, ITP evolves into a chronic disorder in 80% of adults.
- •
Antiplatelet glycoprotein antibodies cause thrombocytopenia through 2 mechanisms: (1) reducing the survival of circulating platelets, and (2) inhibiting the production of new platelets by bone marrow megakaryocytes.
- •
The first line of therapy for ITP includes corticosteroids, sometimes in conjunction with intravenous immunoglobulin or anti–rhesus D. Although these are effective therapies, none reliably induce durable remission.
- •
Second-line therapy for ITP may include rituximab, splenectomy, or thrombopoietin receptor agonists. There is no consensus as to which is superior, and no controlled data to support the preferential use of one rather than the other. Splenectomy provides the greatest chance for long-term remission, but its use is declining.
Definition and history
Immune thrombocytopenia (ITP) is a common hematologic disorder that affects patient of all ages, genders, and races. Initially known as idiopathic thrombocytopenic purpura, an International Working Group (IWG) on ITP recently recommended that this disease be designated immune thrombocytopenia (retaining the abbreviation ITP); this terminology recognizes the immune pathogenesis of ITP and that patients with ITP may not uniformly exhibit purpura or bleeding manifestations. The IWG also proposed terminology to allow standardized disease classification ( Table 1 ). In this scheme, primary ITP is defined as isolated thrombocytopenia (platelet count <100 × 10 9 /L) in the absence of other causes or disorders that may be associated with thrombocytopenia. Secondary ITP is defined as any form of ITP other than primary; this might include thrombocytopenia secondary to systemic lupus erythematosus, hepatitis C infection, or lymphoproliferative disorders. The term acute ITP has been replaced by newly diagnosed ITP, which refers to ITP diagnosed within the preceding 3 months. ITP of 3 to 12 months’ duration is designated as persistent ITP, whereas chronic ITP is defined as disease of more than 12 months’ duration. Severe ITP refers to the presence of bleeding symptoms at presentation, or the development of new bleeding symptoms while on therapy, requiring additional intervention. Refractory ITP designates cases of ITP that have not responded to splenectomy or have relapsed thereafter, and are sufficiently severe or pose sufficient risk of bleeding to require ongoing therapy. Definitions to standardize criteria for responses to ITP therapy have also been proposed.
| Primary ITP | An autoimmune disorder characterized by isolated thrombocytopenia (platelet count <100 × 10 9 /L) in the absence of other causes and disorders that may be associated with thrombocytopenia. The diagnosis of primary ITP is one of exclusion, because no clinical or laboratory parameters are available to establish its diagnosis with accuracy. The main clinical problem in patients with primary ITP is an increased risk of bleeding, although bleeding symptoms are not always present |
| Secondary ITP | All forms of immune-mediated thrombocytopenia other than primary ITP. The acronym ITP should be followed by the name of the associated disease, (eg, secondary ITP–lupus associated, secondary ITP–drug induced) |
| Phases of the disease | Newly diagnosed ITP: within 3 mo of diagnosis |
| Persistent ITP: 3–12 mo from diagnosis. Includes patients not reaching spontaneous remission or not maintaining complete response off therapy | |
| Chronic ITP: lasting for more than 12 mo | |
| Severe ITP: presence of bleeding symptoms at presentation sufficient to mandate treatment, or occurrence of new bleeding symptoms requiring additional therapeutic intervention with a different platelet-enhancing agent or an increased dose |
ITP has probably existed for centuries, and its history has recently been reviewed by Stasi and Newland. Initial descriptions of purpura date to the Greco-Roman era and have been attributed to physicians such as Hippocrates and Galen. The most thorough early description of ITP was from Werhlof in 1735, who described a 16-year-old girl with postinfectious bleeding symptoms including epistaxis and hematemesis. In 1808, Willan described “purpura simplex,” characterized by diffuse petechiae in the absence of systemic symptoms and occurring primarily in women and children. The recognition of platelets as a distinct entity in the blood with an important role in hemostasis is attributed to Bizzozero in 1882, and led to the correlation between purpura simplex and thrombocytopenia, reported by Brohm in 1883. Kaznelson, a medical student, hypothesized that ITP resulted from destruction of platelets in the spleen; this led to the first splenectomy for ITP, performed by Kaznelson’s mentor, Professor Doktor Schloffer, in 1916, inducing complete resolution of severe thrombocytopenia in a 36-year-old woman.
Definition and history
Immune thrombocytopenia (ITP) is a common hematologic disorder that affects patient of all ages, genders, and races. Initially known as idiopathic thrombocytopenic purpura, an International Working Group (IWG) on ITP recently recommended that this disease be designated immune thrombocytopenia (retaining the abbreviation ITP); this terminology recognizes the immune pathogenesis of ITP and that patients with ITP may not uniformly exhibit purpura or bleeding manifestations. The IWG also proposed terminology to allow standardized disease classification ( Table 1 ). In this scheme, primary ITP is defined as isolated thrombocytopenia (platelet count <100 × 10 9 /L) in the absence of other causes or disorders that may be associated with thrombocytopenia. Secondary ITP is defined as any form of ITP other than primary; this might include thrombocytopenia secondary to systemic lupus erythematosus, hepatitis C infection, or lymphoproliferative disorders. The term acute ITP has been replaced by newly diagnosed ITP, which refers to ITP diagnosed within the preceding 3 months. ITP of 3 to 12 months’ duration is designated as persistent ITP, whereas chronic ITP is defined as disease of more than 12 months’ duration. Severe ITP refers to the presence of bleeding symptoms at presentation, or the development of new bleeding symptoms while on therapy, requiring additional intervention. Refractory ITP designates cases of ITP that have not responded to splenectomy or have relapsed thereafter, and are sufficiently severe or pose sufficient risk of bleeding to require ongoing therapy. Definitions to standardize criteria for responses to ITP therapy have also been proposed.
| Primary ITP | An autoimmune disorder characterized by isolated thrombocytopenia (platelet count <100 × 10 9 /L) in the absence of other causes and disorders that may be associated with thrombocytopenia. The diagnosis of primary ITP is one of exclusion, because no clinical or laboratory parameters are available to establish its diagnosis with accuracy. The main clinical problem in patients with primary ITP is an increased risk of bleeding, although bleeding symptoms are not always present |
| Secondary ITP | All forms of immune-mediated thrombocytopenia other than primary ITP. The acronym ITP should be followed by the name of the associated disease, (eg, secondary ITP–lupus associated, secondary ITP–drug induced) |
| Phases of the disease | Newly diagnosed ITP: within 3 mo of diagnosis |
| Persistent ITP: 3–12 mo from diagnosis. Includes patients not reaching spontaneous remission or not maintaining complete response off therapy | |
| Chronic ITP: lasting for more than 12 mo | |
| Severe ITP: presence of bleeding symptoms at presentation sufficient to mandate treatment, or occurrence of new bleeding symptoms requiring additional therapeutic intervention with a different platelet-enhancing agent or an increased dose |
ITP has probably existed for centuries, and its history has recently been reviewed by Stasi and Newland. Initial descriptions of purpura date to the Greco-Roman era and have been attributed to physicians such as Hippocrates and Galen. The most thorough early description of ITP was from Werhlof in 1735, who described a 16-year-old girl with postinfectious bleeding symptoms including epistaxis and hematemesis. In 1808, Willan described “purpura simplex,” characterized by diffuse petechiae in the absence of systemic symptoms and occurring primarily in women and children. The recognition of platelets as a distinct entity in the blood with an important role in hemostasis is attributed to Bizzozero in 1882, and led to the correlation between purpura simplex and thrombocytopenia, reported by Brohm in 1883. Kaznelson, a medical student, hypothesized that ITP resulted from destruction of platelets in the spleen; this led to the first splenectomy for ITP, performed by Kaznelson’s mentor, Professor Doktor Schloffer, in 1916, inducing complete resolution of severe thrombocytopenia in a 36-year-old woman.
Causes and mechanisms of primary and secondary ITP
The pathogenesis of ITP involves loss of tolerance to glycoproteins expressed on platelets and megakaryocytes. ITP is not a single disorder, but a syndrome in which thrombocytopenia may be primary or occur secondary to underlying infectious or immune processes.
Cines and colleagues proposed that the immune tolerance defects in ITP can be divided into 3 categories that include (1) peripheral tolerance defects arising in the setting of immune stimulation, (2) differentiation blocks with skewed peripheral B-cell subsets, and (3) central tolerance defects arising during development or in the bone marrow. Underlying mechanisms associated with each of these may explain the clinical characteristics of individual cases of ITP. ITP resulting from loss of peripheral tolerance is proposed to be platelet specific, more amenable to therapy, and less likely to recur after treatment. In contrast, defects in central tolerance affect multiple cell types, and treated patients are more prone to relapse because of intrinsic autoreactivity.
Secondary ITP
Examples of secondary ITP related to loss of peripheral tolerance include ITP of childhood, which is preceded by a viral-like illness in two-thirds of affected children, and remits spontaneously in 80% of patients. Loss of peripheral tolerance may also underlie the development of secondary ITP caused by vaccines or infectious exposures such as the mumps-measles-rubella (MMR) vaccine (incidence of 1 in 40,000 administrations), Helicobacter pylori infection, and infection with cytomegalovirus (CMV) or varicella-zoster virus (VZV). Perhaps the most common infection associated with ITP is hepatitis C virus (HCV), which is present in up to 20% of ITP cases, with a higher incidence in certain geographic areas. The pathogenesis of HCV-associated ITP may involve activation of B cells, as well as antibodies cross reactive with HCV and platelet glycoprotein (GP) IIIa. Human immunodeficiency virus (HIV) is another well-described cause of ITP; thrombocytopenia results from decreased platelet production caused by infection of megakaryocytes as well as cross reactive antibodies that react with viral proteins and a linear epitope on GPIIIa (amino acids 44–66), causing platelet lysis through generation of reactive oxygen species. The incidence of thrombocytopenia in patients infected with HIV increases with disease progression, and decreases in response to highly active antiretroviral therapy (HAART).
Examples of ITP associated with blocks in differentiation with B-cell skewing include chronic lymphocytic leukemia (CLL), in which thrombocytopenia develops in 1% to 5% of cases and may correlate with poor prognostic markers and decreased survival. Hodgkin disease, non-Hodgkin lymphomas, and large granulocytic leukemia (LGL) are associated with secondary ITP, although ITP develops in less than 1% of cases. ITP may develop in up to 10% of patients with common variable immunodeficiency. The pathogenesis of ITP or other immune disorders such as autoimmune hemolytic anemia that occur in these patients may involve defects in B-cell tolerance checkpoints and/or deficiencies of memory B-cell subsets.
Examples of defects in central tolerance associated with secondary ITP include the autoimmune lymphoproliferative syndrome (ALPS), a disorder linked to defective B-cell and T-cell apoptosis associated with mutations in genes encoding Fas, Fas-L, or other apoptosis mediators such as caspases. Patients develop hepatosplenomegaly and lymphadenopathy, and 20% develop ITP, sometimes in association with autoimmune hemolytic anemia and/or neutropenia. Evans syndrome is characterized by ITP and autoimmune hemolytic anemia. The antiphospholipid syndrome may be associated with ITP in up to one-third of patients, whereas up to 40% of patients with ITP may have antiphospholipid antibodies. The role of antiphospholipid versus antiplatelet glycoprotein antibodies in the development of thrombocytopenia is uncertain, because anti-GPIIIa antibodies have been described in thrombocytopenic patients with antiphospholipid antibodies. ITP develops in up to one-third of patients with systemic lupus erythematosus, which is associated with a broad array of autoantibodies. The management of thrombocytopenia in patients with lupus is difficult, and corticosteroids and splenectomy are less effective than in primary ITP. Defects in central tolerance also develop after transplantation; several mechanisms may be involved, including formation of alloantibodies against donor platelets in the setting of mixed chimerism.
Primary ITP
Like secondary ITP, diverse clinical features and responses to therapy in patients with primary ITP suggest that this apparently more defined disorder also derives from heterogeneous mechanisms. Most patients with primary ITP display a CD4 + Th0/Th1 cytokine profile (associated with increased levels of interferon [IFN]-γ and interleukin [IL]-2) and decreased peripheral Th2 + and T regulatory (Treg) cells. The increased Th1/Th2 ratio may correlate inversely with the platelet count. Alterations in levels of apoptosis regulatory factors in T cells from patients with ITP may influence T-cell subset expression and promote survival of autoreactive T-cell clones. Reversions of Th1/Th2 ratios and normalization of T-cell Vβ spectratyping may follow therapy with rituximab or splenectomy. Likewise, levels of regulatory T cells improve with responses to rituximab and other ITP therapies, including thrombopoietic agents, suggesting a more complex mechanism for rituximab than CD20 + B-cell depletion. These findings are consistent with the hypothesis that autoantibodies in ITP develop as a consequence of T-cell–dependent antigen-driven clonal expansion and somatic mutation. CD8 + Tc (cytotoxic) T cells may also contribute to the pathogenesis of primary ITP by causing platelet lysis through expression of granzymes A and B, Apo1/Fas, and perforin. Cytotoxic T cells from patients with ITP also mediate toxicity toward megakaryocytes, and increased numbers of VLA4 + CD3 + CD8 + T cells expressing the homing receptor CX3Cr1 + have been observed in the bone marrow of patients with ITP.
Antiplatelet antibodies
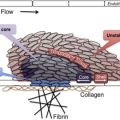
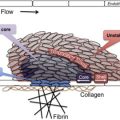
The observations of Kaznelson and Schloffer provided evidence for a central role of the spleen in the pathogenesis of ITP. Other studies showed that injection of an extract of splenic tissue from patients with ITP into rabbits produced a rapid decrease in the platelet count. The studies of Harrington and colleagues in 1951 were the first to provide evidence of a circulating plasma component in the pathogenesis of human ITP. They arranged an exchange transfusion between Harrington and a woman with chronic ITP and a platelet count of 5 × 10 9 /L. Afterward, he developed severe thrombocytopenia that resolved over the next 7 days. Subsequent studies with additional volunteers yielded similar results, although the response to ITP plasma was variable and severe thrombocytopenia developed in only 16 of 26 recipients. In 1965, Shulman and colleagues reported that the thrombocytopenic factor in ITP plasma was a platelet-reactive immunoglobulin (Ig)G antibody. Studies performed in the 1970s showed increased levels of platelet-associated IgG in 90% of patients with ITP, although subsequent work showed that much of this material bound to platelets nonspecifically or was contained within alpha granules. The development of antigen-specific antiplatelet antibody assays in the 1980s identified IgG reactive with platelet surface glycoproteins, primarily GPIIbIIIa (integrin αIIbβ3) and GPIb/IX, in 60% of patients with ITP. Although antiplatelet antibodies may initially be directed toward a single platelet glycoprotein, following uptake of antibody-coated platelets and processing of antigenic peptides from originally nontargeted platelet glycoproteins, the production of antibodies against these new targets may ensue as a result of epitope spreading ( Fig. 1 ).
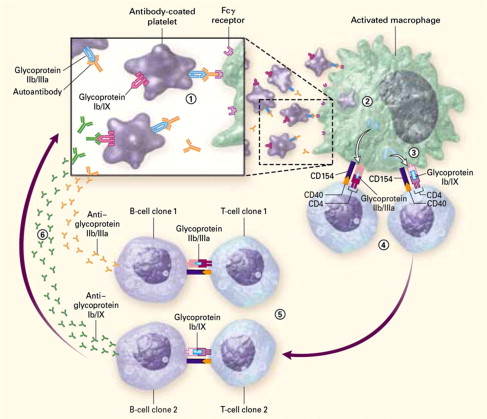
Antibody-dependent mechanisms of platelet destruction
Platelet survival is decreased in patients with ITP, with most platelets cleared in the spleen and liver. Antibody-coated platelets are removed by splenic macrophages through Fcγ receptor–mediated phagocytosis. Polymorphisms in the gene encoding FcγRIII (FcγRIIIA-581 V/V), a subtype of Fcγ receptor, are over-represented in adults and children with ITP; the V/V isoform of this receptor binds IgG1 and IgG3 with greater affinity than the F/F or F/V isoforms. An important role for FcγRIII in humans is also suggested by the ability of a monoclonal antibody to this receptor (mAb 3G8) to increase the platelet count in patients with refractory ITP. Although one group has reported an essential role for FcγRIIa in the therapeutic effect of intravenous (IV) immunoglobulin (IVIg) in preclinical models, this finding has not been consistently reproduced.
Although uptake in the spleen is the primary mechanism by which antibody-coated platelets are cleared in patients with ITP, other mechanisms of platelet destruction exist. The failure of splenectomy in one-third of patients may reflect alternative mechanisms of platelet clearance and/or decreased platelet production. Antiplatelet antibodies from more than half of a cohort of 240 patients with ITP were capable of fixing complement on platelets, and some antiplatelet antibodies induce complement-dependent lysis of platelets in vitro. In patients with HIV, antibodies to platelet GPIIIa amino acids 49 to 66 cause platelet lysis in a complement-independent manner by generation of peroxides through the reduced nicotinamide adenine dinucleotide (NADH)/reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system.
Evidence for decreased platelet production in patients with ITP
The concept of decreased platelet production as a cause of ITP was first suggested by Frank in 1915. Like platelets, megakaryocytes express GPIIb/IIIa and GPIb/IX, which are targets for platelet-reactive autoantibodies. Increased numbers of histologically abnormal megakaryocytes, specifically younger and more immature forms, were noted more than 70 years ago in patients with ITP. Similar abnormalities were observed in the megakaryocytes of healthy individuals infused with ITP plasma, and marked inhibition of megakaryopoiesis was observed in rats treated with antiplatelet serum. Electron microscopic studies have confirmed ultrastructural abnormalities in megakaryocytes in patients with ITP consistent with apoptosis and para-apoptosis; these include cytoplasmic vacuolization, mitochondrial swelling, abnormal chromatin condensation, and increased staining for activated caspase-3.
Chang and colleagues showed that plasma from pediatric patients with ITP that contained anti-GPIb and/or anti-GPIIbIIIa antibodies inhibited the maturation of umbilical cord mononuclear cells into mature megakaryocytes in the presence of thrombopoietin. McMillan and colleagues extended this work, showing that plasma from 12 of 18 adult patients with ITP decreased the production of megakaryocytes from CD34-positive cells: these effects were mediated by IgG, and prevented by adsorption of IgG fractions with immobilized GPIIb/IIIa.
Measurement of platelet turnover rates provides convincing evidence that platelet production is impaired in patients with ITP. Because platelet production is equal to platelet destruction at stable platelet concentrations, and because platelet destruction can be measured through the use of 111 In-labeled platelets, platelet production rates can be estimated. Early thrombokinetic studies suggested that platelet production rates were increased in ITP, although these studies relied on allogeneic platelets labeled with Cr 51 , a less effective platelet label. However, later studies in which allogeneic and autologous platelet survival were studied in the same individuals showed significantly longer survival of autologous platelets, particularly in patients in whom autologous platelet survival exceeded 1 day. Platelet production rates estimated based on these later studies led to the conclusion that platelet production is normal or decreased in most patients with ITP.
Levels of plasma thrombopoietin are not increased in patients with ITP, reflecting that thrombopoietin production occurs in the liver and is largely constitutive. Plasma thrombopoietin levels are regulated primarily via clearance, mediated through binding of thrombopoietin to the thrombopoietin receptor (c-Mpl) on circulating platelets and to bone marrow megakaryocytes. Accelerated clearance of platelets in ITP leads to enhanced metabolism of thrombopoietin, and thrombopoietin production rates do not increase proportionally.
Role of cellular immunity in platelet destruction and impaired platelet production
The maturation of antiplatelet antibodies is T-cell–dependent and antigen-dependent. However, antiplatelet antibodies are only detectable in 60% of patients, and ITP may enter remission despite the continued presence of antiplatelet antibodies ; these observations suggest that antibody-independent mechanisms account for thrombocytopenia in some individuals. In one study, CD8 + cytotoxic T cells from patients with active ITP but without detectable antiplatelet antibodies bound and lysed platelets in vitro, although CD8 + T cells from patients with ITP in remission did not. CD3 + T cells from patients with ITP also showed increased expression of genes that mediate cell-dependent cytotoxicity, including perforin, tumor necrosis factor alpha, and granzymes A and B. Li and colleagues reported that CD8 + T cells also prevented apoptosis of autologous megakaryocytes, which is involved in budding and release of proplatelets.
Clinical features of ITP
Epidemiology
ITP affects patients of all genders, races, and ages. Studies from Scandinavia suggest a prevalence of ITP ranging from 4.6 to 5.3 cases per 100,000 children. In a study that analyzed data from the Maryland Health Care Commission, the prevalence of ITP was 9.5 per 100,000 children aged 1 to 5 years, 7.3 per 100,000 in children aged 6 to 10 years, and 4.1 per 100,000 in children of aged 11 to 14 years. Analysis of the UK General Practice Research Database (GPRD) identified a higher incidence of ITP in boys between the ages of 2 and 5 years (9.7 cases vs 4.7 cases in girls per 100,000 patient years, respectively) compared with the incidence in teenagers between the ages of 13 and 17 years (2.4 cases per 100,000 patient years, with equal sex distribution).
The overall prevalence of ITP in adults is comparable with that in children. A prospective, population-based study of patients older than 16 years with newly diagnosed ITP showed an annual incidence of 1.6 cases per 100,000 patient years; the incidence was slightly higher in women between 45 and 49 years of age but otherwise no gender differences existed. The highest age-specific incidence of ITP was in individuals more than 60 years old. A Scandinavian study identified an ITP incidence of between 2.25 and 2.68 per 100,000 individuals/y; the incidence was greater in women (female/male ratio 1.7) and the elderly. Other studies have suggested a prevalence of ITP in adults ranging from 4.0 to 23.6/100,000 patient-years. A study based on queries of physicians’ offices suggested a higher prevalence of ITP in women less than 70 years old, but a higher incidence in men more than 70 years old.
Clinical Manifestations of ITP
Because ITP is often secondary, a patient with newly diagnosed thrombocytopenia should be evaluated for symptoms associated with disorders causing secondary ITP such as rashes, arthralgias, or serositis associated with systemic lupus; hepatomegaly and increased transaminase levels associated with hepatitis C; or fever and lymphadenopathy associated with infection or lymphoid malignancy. A history of prescription and nonprescription drug intake, including herbs and supplements, is of critical importance.
This section focuses on the manifestations of primary ITP, which may dominate even in cases of secondary ITP.
Bleeding
Bleeding is the most common clinical manifestation of ITP, presenting as mucocutaneous bleeding involving the skin, oral cavity, and gastrointestinal tract. Purpura, usually on the extremities (dry purpura), may often appear without an obvious precipitating event. Mucosal bleeding includes epistaxis, menorrhagia, and gingival and gastrointestinal bleeding. Patients with severe thrombocytopenia may display oral hemorrhagic bullae (wet purpura), which may be a harbinger of more severe bleeding manifestations in the gastrointestinal tract or elsewhere. Bleeding from more distal sites in the gastrointestinal tract may develop at the site of unsuspected preexisting lesions.
Intracranial hemorrhage is the most feared complication of ITP. The incidence of intracranial hemorrhage in children has been estimated to be less than 0.2%, almost always occurring at platelet counts less than 10 × 10 9 /L. A recent report from the International Cooperative Study shows that this complication occurs more frequently in adults than in children, occurring in 10 of 1784 children and 6 of 340 adults with newly diagnosed ITP. In a natural history study that enrolled 152 patients, 4 patients died of ITP-related causes in the first 2 years (1 because of hemorrhage, 3 because of infection), and 2 died of ITP-related causes during long-term follow-up (1 with postsplenectomy sepsis, 1 with refractory bleeding and a platelet count of 2 × 10 9 /L). Patients with ITP may be at increased risk of hematologic malignancy, consistent with the demonstration of an increased frequency of CLL phenotype lymphocytes in patients with ITP.
Several risk factors for bleeding in patients with ITP have been identified. Cohen and colleagues identified 49 cases of fatal hemorrhage in 1718 patients from pooled ITP case series. The overall risk of fatal hemorrhage was between 0.0162 and 0.0389 cases per patient-year, with a risk of 0.004 in patients less than 40 years old increasing to 0.130 for patients more than 60 years of age. Cortelazzo and colleagues observed an overall incidence of hemorrhagic events of 3.2% per patient-year in patients with ITP. Hemorrhagic events at similar platelet counts occurred in 10.4% of patients older than 60 years, compared with 0.4% in patients less than the age of 40 years. A previous history of hemorrhage also predicted bleeding (relative risk [RR] 27.5). Michel and colleagues compared the incidence of bleeding and other outcomes in 55 patients with ITP older than 70 years (mean age 77.8 ± 6.1 years) with those of a younger cohort (mean age 40.3 ± 14.9 years). The median platelet count at diagnosis did not differ between the 2 groups, although bleeding symptoms were more frequent in the older (82%) versus the younger (62%) group.
In a prospective study of 245 patients older than 16 years with newly diagnosed ITP, 30 (12%) presented with frank bleeding, 28% were asymptomatic, and the remainder displayed purpura. Bleeding was uncommon at platelet counts more than 30 × 10 9 /L. However, in this and other studies, a direct correlation between the platelet count and severity of bleeding was not uniform, reflecting the observation that occasional individuals with very low platelet counts exhibit little bleeding, whereas others with platelet counts greater than 30 × 10 9 /L bleed frequently. This conundrum might be explained by binding of some antiplatelet antibodies to highly restricted regions in the GPIIb beta-propeller domain near the ligand (fibrinogen) binding site, potentially interfering with platelet aggregation.
Fatigue
Fatigue is an underappreciated symptom in patients with ITP, occurring in approximately 22% of children, and 22% to 39% of adults. Significant improvements in fatigue and several health-related quality-of-life measurements have been observed in successfully treated patients. In one study, univariate analyses showed that the presence of fatigue correlated with a platelet count less than 100 × 10 9 /L, treatment with steroids, bleeding, and several other factors, but not with duration of ITP, age, or gender. Fatigue in patients with ITP may reflect, in part, increased levels of inflammatory cytokines, including IL-2 and IFN-γ, associated with the Th1 profile.
Thrombosis
Recent studies suggest that patients with ITP have an increased risk of thrombosis. Aledort and colleagues initially reported 18 thromboembolic events in 186 adults with chronic ITP. Sarpatwari and colleagues observed that the adjusted hazard ratio for venous, arterial, or combined thromboembolic events in patients with ITP mined from the UK GPRD were 1.58 (95% confidence interval [CI], 1.01–2.48), 1.37 (95% CI, 0.94–2.00), and 1.41 (95% CI, 1.04–1.91), respectively. The severity of thrombocytopenia correlated with the development of thrombosis. A study using a matched ITP cohort from the Danish National Patient Registry observed an incidence rate ratio for venous thromboembolism in patients with ITP of 2.04 (95% CI, 1.45–2.87).
The mechanisms underlying the paradoxic development of thrombosis in patients with ITP are uncertain. The incidence of antiphospholipid antibodies (APLA) is increased in patients with ITP, and patients with ITP with APLA may develop thrombosis more frequently. Although current guidelines do not recommend routine screening of patients with ITP for APLA, this should be considered in patients who develop thrombosis. Other factors that may contribute to the development of thrombosis include increased levels of prothrombotic, platelet-derived microparticles and complement activation on antibody-coated platelets.
The management of thrombosis in thrombocytopenic patients with ITP is not addressed by current guidelines. Many experts consider anticoagulation to be justified at platelet counts more than approximately 40 × 10 9 L, although this should be individualized depending on the severity of the thrombotic event and characteristics of the patient. Aggressive treatment of ITP is warranted during anticoagulation therapy.
Laboratory studies
ITP is characterized by isolated thrombocytopenia without abnormalities in erythrocyte or leukocyte number or morphology. Platelet size may be normal or increased, although not usually to the degree observed in inherited causes of thrombocytopenia such as MYH9-related macrothrombocytopenias. A careful examination of the peripheral blood smear is essential to exclude other causes of thrombocytopenia such as microangiopathic processes, platelet satellitism, or pseudothrombocytopenia. Myelodysplastic syndromes or acute and chronic leukemias occasionally present with isolated thrombocytopenia, although in many cases review of the peripheral blood reveals characteristic changes in other hematopoietic lineages. A recent report showed decreases in the absolute immature platelet fraction (A-IPF) in patients with ITP. Treatment with eltrombopag increased the A-IPF.
Normal or increased numbers of megakaryocytes are present in the bone marrow of patients with ITP, sometimes with an increase in immature megakaryocytes. Ultrastructural examination may show evidence of megakaryocyte apoptosis.
The sensitivity of measurements of platelet-associated IgG for the diagnosis of ITP is 91%, although the specificity is only 27%; thus, the positive predictive value is only 48% and the diagnostic usefulness of such assays is poor. Measurement of specific platelet glycoprotein antibodies offers greater specificity (78%–92%), although their diagnostic value is limited by low sensitivity (49%–66%) leading to a positive predictive value of only 80% to 83%.
Several other laboratory parameters in the diagnostic evaluation of ITP have been recommended by the ITP IWG ; those considered to comprise the basic evaluation or to be potentially useful are listed in Table 2 . A reticulocyte count and direct antiglobulin (Coombs) test are recommended to exclude concurrent autoimmune hemolytic anemia. Blood type may be useful in determining the usefulness of therapy with anti-Rhesus D [anti-Rh(D)], whereas quantitative immunoglobulin levels may lead to the diagnosis of common variable immunodeficiency. Screening for HIV, HCV, and H pylori is recommended regardless of geographic location, although the importance of H pylori in the development of ITP in North America is not well established.
| Basic Evaluation | Potential Usefulness | Uncertain or Unproven Benefit |
|---|---|---|
| Complete blood count and reticulocyte count | Glycoprotein-specific antiplatelet antibody | Thrombopoietin level |
| Peripheral blood film | Antiphospholipid antibodies | Reticulated platelets |
| Quantitative immunoglobulin level (consider in children, recommend in children with persistent or chronic ITP) | Thyroid function and antithyroid antibodies | Platelet-associated IgG |
| Bone marrow examination (may be informative in patients >60 y old, with systemic symptoms, or before splenectomy) | Pregnancy test (women of childbearing potential) | Platelet survival study |
| Blood group (Rh) | Antinuclear antibodies | Bleeding time |
| Direct antiglobulin test | PCR for parvovirus and CMV | Serum complement |
| H pylori | — | — |
| HCV | — | — |
| HIV | — | — |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree