Chapter Outline
Plain Language Summary 300
Background 300
Selecting an Appropriate Surveillance Regimen 301
Negative Test Results 302
Positive Test Results 302
Incidental Findings 302
Balancing the Benefits and Potential Harms of Surveillance 303
Modalities for Imaging Surveillance 303
Mammography—Association With Survival 303
Mammography Performance 304
DBT for Screening 306
DBT for Posttreatment Surveillance 307
Breast MRI 308
Breast Ultrasound 309
Adherence to Guidelines 310
Breast Cancer Subtypes 312
Knowledge Gaps and Areas for Further Research 316
List of Acronyms and Abbreviations 317
References
Plain Language Summary
Following successful treatment of first breast cancers, women remain at risk of cancer recurrence in the treated breast and also new cancers in the other breast (second breast cancers), which are associated with increased rates of cancer detection in other parts of the body and with death from breast cancer. Imaging surveillance after breast cancer treatment aims to detect second breast cancers before symptoms develop, permitting treatments which may improve survival and maintain quality of life.
This chapter reviews the evidence which supports the use of annual mammography for imaging surveillance in women with a history of breast cancer. Evidence that mammography is underused among breast cancer survivors, especially as time since completing treatment increases, offers an opportunity to improve how mammography can be used to improve survival. In addition, new technologies for detecting breast cancer, such as digital breast tomosynthesis (DBT), breast ultrasound, and breast magnetic resonance imaging (MRI) will be reviewed. DBT, also known as “3D mammography,” uses the same X-ray source as digital mammography and moves in an arc around the breast to collect digital information. By presenting images of thin “slices” within the breast, DBT more clearly shows true breast lesions and reduces false-positive findings due to overlapping normal breast tissue. MRI uses strong magnetic fields to image the breast, rather than ionizing radiation. Its ability to detect cancers is very high, and MRI is recommended for breast cancer screening in women with very strong family histories, especially those who are at very high risk of developing breast cancer due to gene mutations such as BRCA1 and BRCA2 . The benefit of breast MRI use in breast cancer survivors who do not have these risk factors is still being studied. Breast ultrasound uses high frequency sound waves to examine breast tissue, and a strength of ultrasound is its ability to differentiate cystic from solid breast masses. The main limitation of breast ultrasound when it is used for breast cancer screening or surveillance, is the high number of biopsies which are recommended, most of which have benign (not cancer) results.
We are also improving our understanding of cancer biology, and new information about breast cancer subtypes may help to guide treatment selection and improve survival. Information about how breast cancer subtype is related to detection of first and second cancers is needed to help women decide which imaging tests should be used for imaging surveillance. Other factors, such as quality of life, costs of care, and care that takes into account a woman’s individual preferences and values, are also important. It is critical for patients and their doctors to consider these additional factors when making decisions about treatment and imaging surveillance, so that women can lead longer, healthier lives once their treatment is done. This chapter will review the current evidence and highlight critical knowledge gaps where further study is needed to improve clinical care and outcomes.
Background
Globally, 28.8 million individuals alive in 2008 were cancer survivors who had been diagnosed within the last 5 years. When identifying survivors by cancer site, female breast cancer is the most prevalent neoplasm worldwide, at approximately one in six cancer survivors. In the United States alone, approximately 232,000 women were diagnosed with invasive breast cancer in 2013. Advances in screening and treatment of primary breast cancer (PBC) have improved survival for many of these women, and the number of breast cancer survivors will continue to increase. Women who survive their initial diagnosis of breast cancer remain at risk of subsequent local recurrence (LR) and new primary cancers in the contralateral breast (second breast cancers), which are associated with increased rates of distant metastases and breast cancer mortality.
Posttreatment surveillance aims to detect asymptomatic second breast cancers, permitting interventions to potentially improve survival and maintain quality of life. Based on randomized controlled trials (RCTs) demonstrating the effectiveness of screening mammography in reducing breast cancer mortality (see also chapter: Estimates of Screening Benefit: The Randomized Trials of Breast Cancer Screening ) and on observational studies suggesting effectiveness for posttreatment surveillance, current guidelines are consistent in their recommendations supporting the use of mammography in women following treatment for PBC.
With advances in breast imaging technology, additional options beyond mammography are now available for screening and surveillance. These modalities include DBT, as well as breast ultrasound and MRI. In addition, improvements in our understanding of tumor biology, and specifically in the classification of tumors by gene expression profiles or “molecular subtypes,” are providing independent prognostic information to guide tailored selection of breast cancer treatment. The provision of clinical care is increasingly focusing on patient centered outcomes beyond survival, such as quality of life, costs of care, and shared decision making based on patient preferences and values.
This chapter will consider these three recent trends influencing imaging surveillance regimen selection.
Selecting an Appropriate Surveillance Regimen
Surveillance testing extends beyond application of the test itself. Rather, it is an episode of care that begins with the surveillance test and also includes the subsequent cascade of diagnostic evaluation of positive test results and incidental findings, as well as treatment of the targeted disease. Achieving an overarching balance of benefits and harms with surveillance includes consideration of events within entire episodes of care ( Fig. 12.1 ).
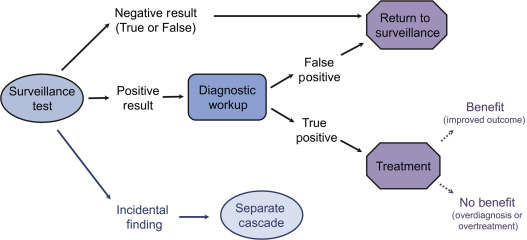
Negative Test Results
A highly specific surveillance test, one which correctly excludes patients without the targeted disease, is an essential characteristic of a surveillance test. Ideally, these patients with true negative results will gain reassurance that they do not have disease recurrence. When recurrence is present but the test result is negative (false-negative), the disease will continue to progress until it presents symptomatically, and the patient will not have gained the benefits of earlier detection. A negative surveillance test could potentially provide false reassurance and cause an individual to delay seeking care for a symptom, causing further harm.
Positive Test Results
Because a population under screening or surveillance has no signs or symptoms of disease, it is important to consider the diagnostic consequences of a positive test result. An important potential harm of surveillance is false-positive results. Individuals with false-positive results will incur all the subsequent diagnostic consequences of a positive test—invasive biopsies, increased costs, and anxiety about having a life-threatening recurrence—but gain none of the benefits, as recurrent disease is not present. The likelihood of having false-positive results increases with an increased surveillance timeframe or with more frequent surveillance intervals, both of which increase the total number of lifetime surveillance episodes, and also with decreasing disease prevalence. Because of these factors, the potential harms of false-positive surveillance results are most likely to accrue in individuals at lower risk of having recurrent disease.
A highly sensitive surveillance test should detect most patients with the targeted disease (a high true-positive proportion), enabling earlier diagnosis of recurrence and access to effective treatments. These patients are the only ones with the potential to benefit from surveillance and to achieve improved long-term outcomes, such as reduced breast cancer mortality and increased overall survival.
Even with a highly sensitive test, an important consequence of screening and surveillance is the potential for harm from overdiagnosis and overtreatment. Overdiagnosis and overtreatment occur when surveillance testing detects asymptomatic disease that would not have become clinically apparent over an individual’s lifetime, or when detection results in treatment of disease that would not have shortened an individual’s life expectancy (see also Challenges in Understanding and Quantifying Overdiagnosis and Overtreatment, Treatment of Screen-Detected Breast Cancer: Can We Avoid or Minimize Overtreatment? ). Both of these scenarios occur more frequently when older populations with higher competing mortality risks undergo surveillance. Due to the invasiveness of treatment and associated morbidity, overdiagnosis and overtreatment may be among the most significant potential harms associated with surveillance.
Incidental Findings
Incidental findings, discovered during image interpretation that are unrelated to the indication of the study, are a routine part of diagnostic radiology. In examinations with larger fields of view, such as breast MRI, there is potential for the discovery of incidental findings that are unrelated to the targeted disease. For example, Niell et al. reported that of 2324 patients receiving breast MRI examinations performed for any indication, 86 patients (3.7%) had extramammary findings for which additional imaging evaluation was recommended, and nine patients (0.4%) had clinically important findings. Potential harms from incidental findings may result from complications of unnecessary invasive procedures, increased costs, and undue patient anxiety for what is ultimately determined to be a benign lesion. These “incidentalomas” place patients and their providers in a difficult situation, as it may not be possible to predict which will be clinically significant and which will not at the time of examination interpretation.
Balancing the Benefits and Potential Harms of Surveillance
The burden of proof for the effectiveness of screening and surveillance is higher than that for diagnostic tests and treatments. Of all the individuals who will undergo surveillance, only those with true-positive test results who receive effective treatment and are not overdiagnosed/overtreated will obtain its primary benefits, which include decreased morbidity and mortality. As we increasingly focus on providing value-based healthcare, with the goal of improving patient outcomes while maintaining or decreasing healthcare costs, the important outcomes to measure extend beyond those of mortality reduction. Outcomes that matter are condition-specific and multidimensional—including overall survival, time without symptoms, quality of life, and the financial consequences of choosing alternative treatments. No single outcome will fully capture the effects of surveillance. Implementation of new imaging-based surveillance tests in clinical practice will increasingly hinge on the successful conduct of research on a scale and with a level of rigor not seen in the past.
The challenge and opportunity for future research studies of imaging-based surveillance are to provide evidence on multiple outcomes to characterize not only its benefits but also its downstream consequences and potential harms. Improved quality of life or reduced disease-specific mortality must be considered in the context of radiation-induced risks, utilization of additional resources to evaluate incidental findings, false-positive test results, and the potential for overdiagnosis and overtreatment. The budget impact and cost-effectiveness of new surveillance regimens are also important factors for which examination prior to adoption and implementation should be considered.
Modalities for Imaging Surveillance
Mammography—Association With Survival
The primary modality for breast cancer screening is mammography—a fast, noninvasive, relatively inexpensive screening test that has been shown in RCTs to reduce breast cancer mortality in women ages 40–74 (more detailed discussion in chapter: Estimates of Screening Benefit: The Randomized Trials of Breast Cancer Screening ). While the benefit of surveillance mammography to detect asymptomatic second breast cancers in women with a personal history of treated breast cancer is presumed, no RCTs comparing surveillance strategies have been conducted. The evidence support for surveillance mammography is based on the established mortality reduction from screening mammography trials, and also from results of observational studies. A review of the medical literature reported that studies of mammographic surveillance varied in terms of study design, focusing on asymptomatic versus symptomatic detection of second breast cancers, mammographic versus clinical detection, or routine versus nonroutine follow-up. Across varying study designs, their results were consistent in reporting that earlier detection of second breast cancers was associated with reduced mortality.
In three studies evaluating survival from the time of PBC diagnosis, which minimizes lead time bias due to earlier diagnosis of second breast cancers with surveillance, the results were consistent. The largest study of 1044 consecutive women with a second breast cancer, compared asymptomatic detection versus symptomatic detection of second breast cancers. The hazard ratio (HR) for breast cancer mortality after asymptomatic detection of second breast cancer, adjusted for length bias, was 0.53–0.73. In another study of women with contralateral breast cancer (CBC; n = 656) and matched controls, the HR for breast cancer mortality after mammographic detection versus other means of detection was 0.31 (95% CI (confidence interval): 0.16–0.63). A study comparing exposure to surveillance mammography to no surveillance reported an HR for breast cancer mortality with mammography exposure to be 0.22–0.37, after adjusting for age, stage, and therapy received.
A metaanalysis of the impact of early detection of second breast cancers on survival reported improved survival for women if a recurrence was found by surveillance mammography compared with clinical detection (HR =2.44, 95% CI: 1.78–3.35) or if a recurrence was detected in asymptomatic women compared to those with symptoms (HR =1.56, 95% CI: 1.36–1.79). Based on a pooled HR of 1.68 for earlier detection, the authors further estimated that the absolute breast cancer mortality reduction achievable was 17–28%.
Current clinical guidelines issued since 2010 for imaging surveillance after treatment of PBC are consistent in their recommendation of annual surveillance mammography for at least the first 5 years following treatment ( Table 12.1 ). Organizations supporting surveillance mammography include the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (United States), the American Cancer Society, the American College of Radiology, the Society of Breast Imaging, the European School of Oncology (ESO), and the National Institute for Health and Care Excellence (NICE, United Kingdom). While the organizations based in the United States recommend annual surveillance mammography without specifying a milestone for cessation, the ESO recommends a 1 to 2-year surveillance interval for postmenopausal women, and NICE bases surveillance in posttreatment year 6 and beyond on patient age at diagnosis and “patient risk category.”
| Organization | Imaging Surveillance Recommendation | |
|---|---|---|
| American Society of Clinical Oncology | Women who have undergone breast-conserving surgery |
|
| National Comprehensive Cancer Network |
| |
| American Cancer Society |
| |
| American College of Radiology/Society of Breast Imaging |
| |
| ||
Not recommended:
| ||
| European School of Oncology (ESO) | Premenopausal women |
|
| Postmenopausal women |
| |
Note:
| ||
| National Institute for Health and Care Excellence (NICE, United Kingdom) |
| |
| International Agency for Research on Cancer (IARC) | Women with personal history of breast cancer |
|
Additional evidence supports the effectiveness of adherence to clinical guidelines. In a series of cohort studies, the most recent being a study of 1846 women with stage I and II PBC who were at least 65 years old receiving care in six integrated health care delivery systems with complete ascertainment of surveillance mammograms, receipt of any surveillance mammography was associated with reduced breast cancer mortality. Further, each additional surveillance mammogram received was associated with a 0.69-fold decrease in the odds of breast cancer mortality (95% CI: 0.52–0.92).
Mammography Performance
A systematic review of surveillance mammography test performance from 1990 to 2006 identified two studies which reported sensitivities of 64% and 67%, and specificities of 97% and 85% for film mammography performance. Both studies had relatively small sample sizes of 83 and 105 patients, respectively. A more recent study of surveillance mammography accuracy was conducted within the Breast Cancer Surveillance Consortium, where multiple mammography registries across the United States are linked with tumor registries. Examining 58,870 mammograms in 19,078 women with a personal history of breast cancer (American Joint Committee on Cancer stage I or II), Houssami et al. reported more robust and also similar results. While the cancer detection rate was higher in women with a prior history of breast cancer (PHBC) compared to women without this history (6.8 vs 4.4 per 1000 screens, respectively) the accuracy of surveillance mammography was significantly lower (all comparisons p < 0.001 except as noted). In women with PHBC, sensitivity was 65.4% and specificity was 98.3%. In a matched cohort of women in the same registries without PHBC, sensitivity of screening mammography was 76.5% and specificity was 99.0%. Sensitivity of surveillance mammography was also lower in the initial 5 years (60.2%) than after 5 years from primary cancer treatment (70.8%), and similar for detection of in-breast recurrence (66.3%) and contralateral second cancers (55.1%, p = 0.96).
Two subsequent studies, also conducted within the Breast Cancer Surveillance Consortium, evaluated risk factors for interval invasive second breast cancer presentation after negative surveillance mammography. Houssami et al. identified age at PBC diagnosis <40 years (OR =3.41), extremely dense breasts on mammography (OR =2.55) and treatment with breast conservation without radiation (OR =2.67) as significant predictors of an interval invasive breast cancer within 1 year of a negative surveillance mammogram. Because approximately 70% of in-breast recurrences occur within 5 years of treatment, Lee et al. estimated the 5-year risk of interval invasive second breast cancers. From a cohort of 15,114 women with 47,717 surveillance mammograms diagnosed with American Joint Committee on Cancer stage 0–II unilateral breast cancer from 1996 to 2008, 325 surveillance detected and 138 interval invasive second breast cancers were identified. The cumulative incidence of second breast cancers after 5 years was 54.4 per 1000 women. The 5-year risk of interval invasive second cancer was based on four independent predictors: PBC grade (OR =1.95), PBC mode of detection (OR =2.01 for interval PBC presentation), treatment with breast conservation without radiation (OR =3.27) and heterogeneously dense breasts on mammography (OR =1.54). The range of risk for women varied substantially, from 0.07% to 6.11%.
These results suggest that aggressive tumor biology in a woman’s first breast cancer, as demonstrated by higher grade or clinical presentation after negative screening mammography, may continue to mediate her subsequent surveillance outcomes. Additional factors related to PBC diagnosis and treatment—increased breast density, breast conservation treatment without radiation therapy—contribute to variation in subsequent interval second breast cancer risk.
It is possible that the selective application of adjunctive testing, based on factors known at the time of PBC diagnosis and treatment, to supplement surveillance mammography, either with a more frequent surveillance interval or with another modality, such as breast MRI or ultrasound, could reduce interval invasive second breast cancers. Further studies are needed to examine the potential contribution of supplemental modalities or alternative regimens to improving surveillance outcomes.
DBT for Screening
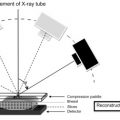
DBT is an innovation upon digital mammography. DBT was designed to eliminate overlapping breast tissue which may obscure breast cancers on standard mammography, when a three-dimensional breast is projected onto a two-dimensional image plane. Using the same X-ray source as digital mammography, a DBT unit moves in an arc around the breast to acquire information from a volume of breast tissue. Computational processing of the digital data reconstructs high-resolution images of thin 0.5–1.0 mm “slices” through the breast tissue at doses similar to standard digital mammography. By presenting images of specific planes within the breast, DBT enables clearer visualization of true breast lesions and reduces false-positive findings due to superimposition of adjacent normal breast tissue. DBT images can be obtained in combination with standard digital mammography, or the digital DBT data can be reconstructed to produce a “synthetic” mammographic view along with the thinner slice images, eliminating the need for a standard digital mammogram and reducing exposure to ionizing radiation.
Studies evaluating DBT compared with digital mammography for breast cancer screening are discussed in greater detail in chapter “Evolution of Mammography Screening: From Film Screen to Digital Breast Tomosynthesis.” Overall, across varying study designs and locations, the studies of DBT for breast cancer screening support decreased recall rates and stable to increased cancer detection, with increased detection more apparent for studies conducted with biennial screening regimens. The primary benefit at the population level is reduction of false-positive screening results and avoidance of diagnostic evaluations in the subsequent cascade of care. With incomplete follow-up in reports to date, and with early reporting of DBT used primarily in prevalence screening, it remains unclear whether the stable to increased cancer detection with DBT will persist with its use in subsequent incidence screening. In addition, it is not yet clear whether the detection of early cancers with DBT will result in improved patient outcomes such as reduced breast cancer mortality and improved overall survival, or whether some overdiagnosis and overtreatment of breast cancers has occurred. Both scenarios are likely, and additional studies with longer-term follow-up are warranted to confirm the presumed benefit of screening with DBT.
DBT for Posttreatment Surveillance
The early reports of DBT performance have evaluated women presenting for routine breast cancer screening in various settings. While there is consistency of results in demonstrating decreased recall rate from screening and probable increase in cancer detection, these characteristics are associated with the underlying prevalence of breast cancer in the population being screened. Sensitivity and specificity, test performance measures which are independent of disease prevalence, have yet to be reported for most studies, due in part to the need for complete follow-up for identification of interval breast cancers.
The studies to date have not focused on DBT performance within subgroups, based on patient demographics or risk factors, such as family history of breast cancer, personal history of breast cancer, or mammographic density. The Oslo study included women invited to undergo routine biennial screening as part of the Oslo, Norway screening program. The only exclusions reported were disabled women unable to stand and women with breast implants. The STORM trial focused on asymptomatic women at standard population risk for breast cancer in Italy. The sites in the Friedewald et al. study in the United States focused on examinations performed for a screening indication, without further specification of population characteristics. Lourenco et al.’s retrospective single institution analysis similarly studied examinations performed for a screening indication. While none of the studies reported excluding women with a personal history of breast cancer, this specific population of women was not the focus of any of the screening studies reported to date.
It is possible that the improved performance of DBT compared with DM will also be observed in women with a personal history of breast cancer. However, given that the accuracy of mammography in women is decreased in women with prior breast cancer compared to women without this history, it is not yet clear whether and to what extent performance improvements with DBT will offset the known performance limitations of mammography for these women. Further studies are needed to evaluate DBT performance for women at varying risk levels and with varying underlying risk factors, including personal history of breast cancer.
Current guidelines do not discuss DBT separately from mammography ( Table 12.1 ).
Breast MRI
MRI uses strong magnetic fields to image the breast, rather than ionizing radiation. Because the detection of breast cancer by MRI is based on changes related to cancer neovascularity, intravenous contrast is a required component of the examination. The sensitivity of breast MRI is not limited by mammographic breast density, and studies of breast MRI for supplemental screening women at increased genetic or familial risk of breast cancer have demonstrated high sensitivity, ranging from 68% to 100%. Warner et al.’s metaanalysis of studies reported sensitivity of 75–77% and specificity of 86–93%. Of the eleven studies included for analysis, nine included women with a PHBC in addition to genetic and familial risk factors for breast cancer. Based on these high-risk screening studies, the American Cancer Society and National Comprehensive Cancer Network recommend breast MRI for supplemental screening women with lifetime risk of breast cancer >20%, with use of familial risk assessment models.
However, women with sporadic breast cancer were not included in these study populations. While it is likely that surveillance MRI has an improved ability to detect asymptomatic second breast cancer compared with mammography, current evidence is limited, based on relatively small single institution studies. Brennan et al. performed a retrospective review of 144 women with prior breast cancer who received breast MRI for surveillance. Of these women, 44 (31%) underwent biopsy prompted by MRI, and 17 women (12%) were subsequently diagnosed with second breast cancer (cancer detection rate 118 per 1000 examinations). The cancer yield of biopsy (positive predictive value) was 39%.
Schact et al. performed a retrospective review of 208 women with prior breast cancer, of which MRI surveillance detected 6 second breast cancers (cancer detection rate 29 per 1000 examinations). When compared with 345 women receiving screening MRI with family history as the sole risk factor, the cancer detection rate was similar, at 20 per 1000 examinations (7/345).
Gweon et al. studied 932 surveillance breast MRI examinations in 607 women after breast conservation therapy (BCT) in Korea, with MRI performed at the request of clinicians or patients. The median age of women receiving MRI surveillance was 48 years (range 20–72 years). Ten second breast cancers were identified by MRI. The cancer detection rate for prevalence imaging was 18 per 1000 women (95% CI: 16–21 per 1000 women). The cancer yield of biopsy performed (PPV3) was 44% (10/23). Multivariable analysis identified age younger than 50 years at initial diagnosis and greater than 24-month interval between initial surgery and screening MRI as independent predictors of MRI-detection of second breast cancer ( p < 0.02 for both factors).
Giess et al. evaluated 1194 MRI examinations in 691 women with a personal history of breast cancer, in which 12 second breast cancers were detected by MRI surveillance. The median age of women receiving MRI surveillance was 47 years. The cancer detection rate for MRI was 10 per 1000 examinations (95% CI: 5–18 per 1000), and the average time from PBC diagnosis to second breast cancer detection was 6.2 years (range 1–23 years). Eight of the twelve surveillance detected second breast cancers were in the ipsilateral breast, and four were new primary cancers in the contralateral breast. The cancer yield of biopsy performed (PPV3) was 18% (12/67).
Lehman et al. compared the performance of breast MRI in 915 asymptomatic women with a personal history of breast cancer, and 606 women with a genetic or family history of breast cancer (GFH). The cancer detection rates in both groups were comparable (20 per 1000 examinations in both groups, p > 0.99). The cancer yield of biopsy (PPV3) was greater in women with a personal history (25%) compared with women with GFH (15%), but the difference was not significant ( p = 0.19). Sensitivity was comparable in both groups of women (80% for personal history and 79% for GFH, p > 0.99) and specificity was higher in women with a personal history (94%, compared with 86% in women with GFH, p < 0.001). The improvement in specificity for women with prior breast cancer was attributable in part to having a prior breast MRI available for comparison, either at the time of first cancer diagnosis to evaluate extent of disease, or a prior surveillance MRI.
While these results suggest that the performance of breast MRI for posttreatment surveillance is comparable to that of MRI for screening women at increased genetic and familial risk of breast cancer, the variation in reported cancer detection rates may be related to relatively small sample sizes for surveillance cohorts, as well as differential selection of women to receive MRI surveillance across studies. Further studies of MRI performance and outcomes in women with a personal history of breast cancer are warranted to guide the appropriate use of breast MRI.
Currently, the American Cancer Society does not recommend for or against breast MRI use for posttreatment surveillance, recommending instead that these women talk with their doctors about the benefits and limitations of adding MRI to the yearly mammogram. The National Comprehensive Cancer Network and European School of Oncology do not specify recommendations regarding breast MRI. ASCO and the NICE (United Kingdom) both recommend against breast MRI for routine surveillance, while the American College of Radiology and Society of Breast Imaging support MRI use in selected patients based on risk assessment.
Breast Ultrasound
Breast ultrasound employs high frequency sound waves to evaluate specific findings identified by physical examination or mammography. It is widely used primarily in the diagnostic setting, and a strength of ultrasound is its ability to differentiate cystic from solid breast masses. Advantages of ultrasound include the ability to evaluate tissue without ionizing radiation exposure associated with mammography, and the lack of breast compression when hand-held ultrasound (HHUS) transducers are used. Automated whole breast ultrasound (ABUS) is a relatively new technology that standardizes imaging acquisition using an automated transducer, rather than a hand-held one, and allows simultaneous visualization of a volume of breast tissue in multiple planes. Whole breast ultrasound is considered an appropriate supplemental screening test in women who are at high risk for developing breast cancer and cannot receive a breast MRI examination, and has been studied for supplemental screening in women with mammographically dense breasts.
The main limitation of breast ultrasound for screening, either HHUS or ABUS, is the high number of false-positive findings, which often lead to biopsies ultimately demonstrating benign disease. Image acquisition time, which is longer than that of screening mammography, is a limitation for both HHUS and ABUS, and operator dependency is an additional limitation of HHUS. ABUS requires breast compression while the patient lays supine, and multiple views of each breast are required in a standardized screening examination.
Most studies of breast ultrasound as a supplemental screening modality have focused on women with mammographically dense breasts, with varying additional risk factors, such as family history or personal history of breast cancer. Of 12 screening ultrasound studies conducted since 2000, the American College of Radiology Imaging Network (ACRIN) 6666 study was the only prospective study evaluating the combination of mammography plus HHUS compared mammography alone, with 1 year follow-up for multiple screening rounds. In this study, 1426 of 2659 women (53%) had a personal history of breast cancer.
In the first screening round conducted from 2004 to 2006, the cancer detection rate for mammography alone was 7.6 per 1000 examinations, and mammography/HHUS detected an additional 5.3 cancers per 1000 examinations. However, the number of recalls for additional evaluation increased 2.3-fold, from 115 per 1000 examinations for mammography alone to 266 per 1000 examinations for mammography/HHUS. The number of breast biopsies increased 4.2-fold, from 24 per 1000 examinations with mammography to 102 per 1000 with mammography/HHUS. The cancer yield for biopsies performed (PPV3) for HHUS was 8.6%, indicating that greater than 90% of biopsies performed revealed benign disease.
By the third screening round, the mammography examinations had transitioned from film to digital, and mammography alone detected 9.9 cancers per 1000 examinations. Mammography plus ultrasound detected an additional 4.2 cancers per 1000. For women with a personal history of breast cancer, the incremental cancer detection rate for HHUS was the same as in women without this history. The recall rate in the second and third screening rounds remained high, at 94 per 1000 examinations for mammography alone and 168 per 1000 for mammography/HHUS, a 1.8-fold increase. Breast biopsies remained increased with mammography/HHUS screening (3.5-fold increase compared with mammography alone), and the cancer yield following biopsy remained low at 7.1%.
While breast ultrasound may detect additional breast cancers not seen on mammography in women with treated breast cancer, the risk of false-positive biopsies as a diagnostic consequence is far greater. No current guidelines support the use of breast ultrasound, either HHUS or ABUS, for supplemental surveillance in women with treated breast cancer ( Table 12.1 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree