Fig. 12.1
Categories of breast by density. A Breasts are almost entirely fatty. B There are scattered areas of fibroglandular density. C The breasts are heterogeneously dense, which may obscure small masses. D The breasts are extremely dense, which lowers the sensitivity of mammography
Approximately 10% of women have entirely fatty breasts (category A), 40% of women have scattered densities (category B), 40% have heterogeneously dense breasts (category C) and 10% of breasts are extremely dense (category D) [7]. Density tends to decrease with age, therefore mammography is more sensitive in women over 40 years of age, but there are women with dense breasts even at an advanced age as well as young women with fatty breasts [8].
The proportion of women with different breast densities in different age groups and sensitivity and specificity of mammography in various patient groups is summarised in ◘ Table 12.1 [9].
Age | A (%) | B (%) | C (%) | D (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
40–49 | 3 | 23 | 57 | 17 | 65.6–69.7 | 90.7–90.9 |
50–59 | 6 | 37 | 49 | 8 | 72.9–73.8 | 91.6–92.3 |
60–69 | 12 | 43 | 41 | 4 | 73.3 | 93 |
70–79 | 13 | 51 | 32 | 4 | 81.4 | 94.1 |
≥80 | 13 | 46 | 34 | 7 | 86.1 | 94.3 |
Breast implants | 45 | 97.7 |
12.1.4 Evaluation of the Image
Evaluation of a mammography study includes assessment of both views of both breasts to detect possible abnormalities; special attention is paid to the axilla and skin. Two views are vital for differentiation of summation artefacts from true lesions. If an asymmetric density is visible only in one view, it is most likely caused by superimposition of normal breast tissue; real lesions typically appear in both views.
Comparison of new and previous images is essential for detecting pathology, especially in the screening setting, where a subtle change of focal breast density may be the first and only sign of malignancy [10] (◘ Fig. 12.2). In benign changes, comparison with previous images confirms the stable character and size of masses, distortions or microcalcifications especially if they are of not-typically benign morphology. In such cases, the patient can be spared unnecessary additional imaging or biopsy [11] (See ◘ Table 12.2).
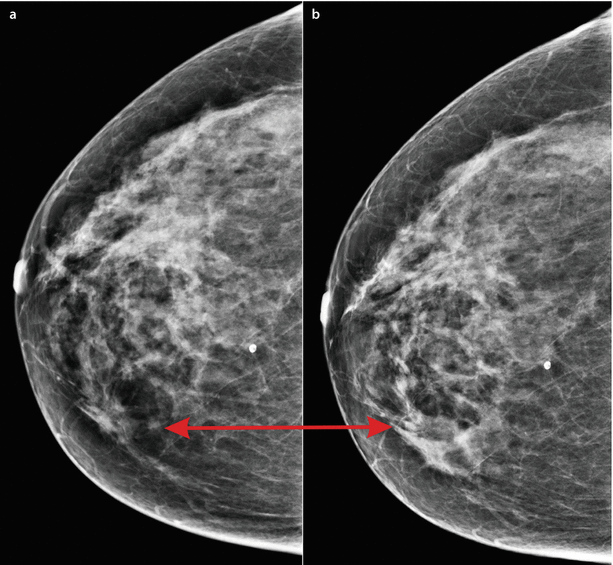

Fig. 12.2
Detecting malignancy. A developing density as the only sign of pathology, visible only in comparison of current b to prior a image
Table 12.2
Factors improving mammography performance
Full-field digital mammography |
Correct positioning of the breast |
Lower breast density |
Comparison with previous images |
The position of the lesion in the breast is determined using two views. As the MLO view is obtained at a 45° angle, the apparent position of the lesion in the MLO view can appear to be shifted from the actual position in the breast. Lesions located laterally are seen more cranially in the MLO image, and lesions with a medial location in the breast are projected more caudally as demonstrated in ◘ Fig. 12.3.
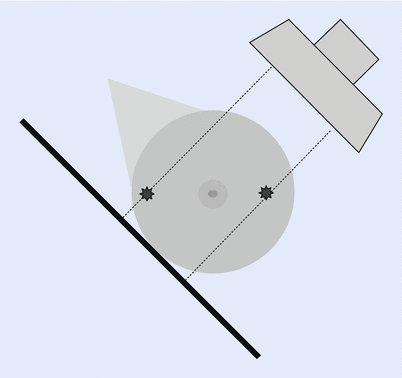

Fig. 12.3
Shift in the apparent position of the lesion. The lesion located laterally is seen more cranial in the image, lesion in medial part of the breast is projected more caudally
12.1.5 Abnormal Findings on Mammography
Abnormal findings on mammography may include:
Masses (characterised by shape, margins and density)
Architectural distortions
Asymmetries
Microcalcifications (evaluated by their morphology and distribution)
Associated features such as skin and nipple changes (thickening, retraction), chest wall invasion or axillary lymphadenopathy may also be seen.
Typical Imaging Findings
Typical malignant lesions appear on mammography as irregular masses with spiculated or indistinct margins and a density equal to or higher than the breast parenchyma; see ◘ Fig. 12.4. Distortion of the parenchyma or retraction may be a sign of malignancy even if a distinct mass is not visible.
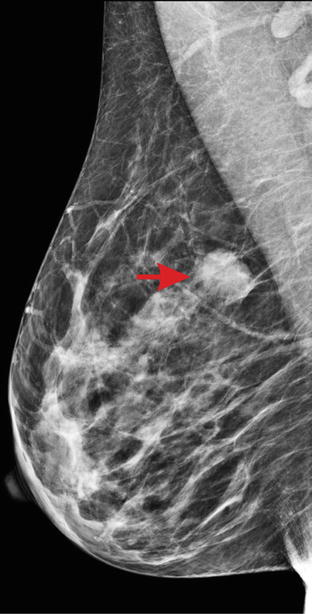

Fig. 12.4
Breast cancer in mammography. Typical appearance of a malignant lesion (arrow) with indistinct margins and higher density than normal parenchyma
Mammography shows higher sensitivity for ductal carcinoma (79.1–80%) than for lobular carcinoma (67.2–72.1%) [12, 13]. In BRCA mutation carriers, the tumours manifest less frequently as spiculated masses or distortions, and the lesions are frequently well circumscribed mimicking benign disease which results in lower sensitivity of mammography in high-risk women [14].
Some malignant lesions manifest mainly as microcalcifications. They may or may not be associated with masses. Microcalcifications are highly suggestive of malignancy if they are amorphous, fine pleomorphic, fine linear with/without branching or coarse and heterogeneous. Distribution following ductal anatomy (linear, segmental or regional) is a suspicious feature (◘ Fig. 12.5) [15].
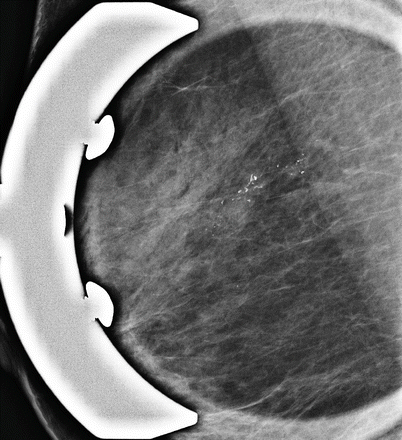

Fig. 12.5
Suspicious microcalcifications in magnification view. Coarse heterogeneous morphology with ductal distribution, histology – ductal carcinoma in situ
Benign lesions are usually oval or round with smooth margins and variable density. Lesions of very low density containing fat are almost always benign. Mammography alone cannot differentiate between solid and cystic lesions as they appear similar; consequently, additional evaluation by ultrasound is usually needed.
Benign microcalcification in the breast is a frequent finding. Benign features include a cutaneous or vascular location, a round shape or rim appearance, large coarse «popcorn-like» morphology typical for calcifying fibroadenomas, a large rod-like shape and a peri-, intraductal or diffusely homogeneous punctate pattern. Amorphous dystrophic calcification after trauma or surgery and milk-of-calcium sediment calcifications in microcysts also have a typical appearance and are of benign origin (◘ Fig. 12.6).
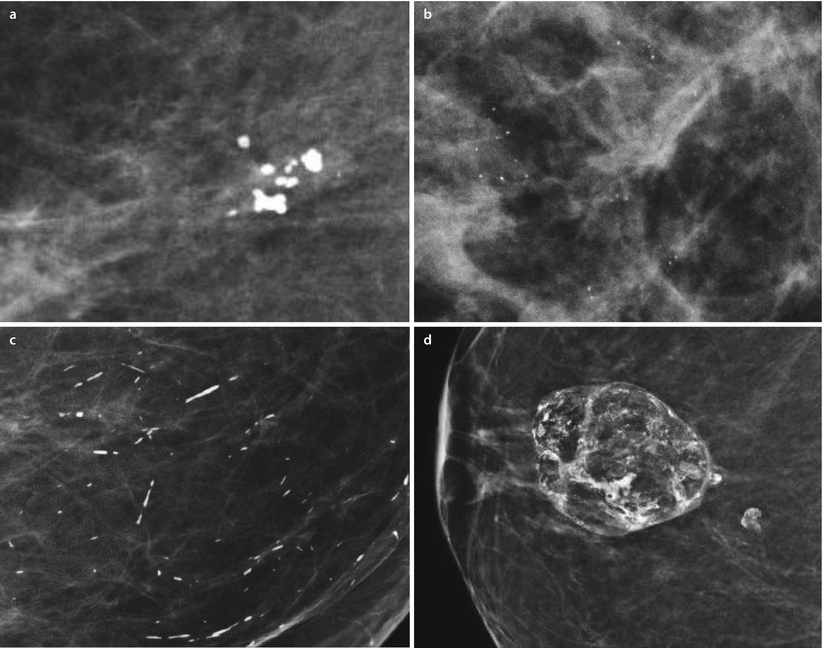

Fig. 12.6
Benign microcalcifications. a coarse «popcorn-like» in a benign mass representing fibroadenoma, b homogeneous punctate microcalcifications, c large rod-like calcifications, d amorphous dystrophic calcifications in fat necrosis after surgery
12.1.6 Evaluation of a Previously Treated Breast
During the follow-up period after locoregional treatment (surgery and radiotherapy), mammography is an essential part of evaluation of the breast to rule out local recurrence [16]. Evaluation of the treated breast can sometimes be challenging due to the presence of post-treatment changes which may make interpretation difficult (◘ Fig. 12.7). It is therefore vital that the request for post-operative imaging also gives details of the treatment.
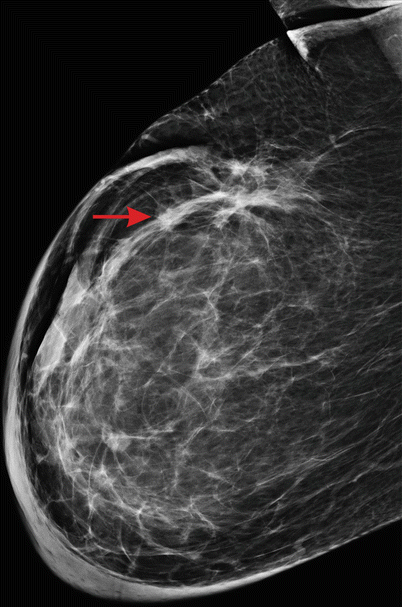

Fig. 12.7
Breast after surgery and radiotherapy. A marked skin thickening and multiple distortions are seen; the recurrence (arrow) is difficult to detect in the altered background
Postsurgical and postirradiation changes include:
Scarring – deformity of the breast, distortion of the parenchymal pattern.
Skin thickening.
Radiation fibrosis and lymphoedema giving rise to a reticulated pattern which may increase the density of the breast parenchyma diffusely.
Postsurgical fluid collections (seromas) and hematomas – may mimic masses and may persist for many months or even years after surgery.
Fat necrosis – especially in cases of conservation where extensive oncoplastic parenchymal mobilisation was performed. Locating the site of the primary tumour may be challenging and scarring within the breast widespread.
Calcification – dystrophic calcification must be differentiated from residual calcifications (not removed by surgery) or recurrent disease.
Artificial material – surgical clips, sutures, implants and autologous flaps.
Lipomodelling – may have been performed which may result in scarring, fat necrosis and calcification.
12.1.7 Future Perspectives
Several new methods based on mammography are being developed and researched to improve diagnostic performance of the modality.
Digital breast tomosynthesis (DBT) is a relatively recent advance providing a 3D picture of the breast. Multiple low-dose images are taken during movement of the x-ray tube in an arc around the breast. A 3D image of the breast is reconstructed from the acquired images and viewed in multiple parallel sections (slices) 1 mm thick. This enables better visualisation of distortions and masses as shown in ◘ Fig. 12.8. DBT has demonstrated increased sensitivity (90.7% vs. 85.2%) in comparison to digital mammography [17] and reduced false positives caused by summation of normal breast structures, asymmetries and distortions [18]. However, evaluation of microcalcification may be limited. Due to the increased number of images, the reading time for a DBT study is longer than for mammography. The radiation dose from DBT (when used as standalone method) is not higher than that of digital mammography [19].
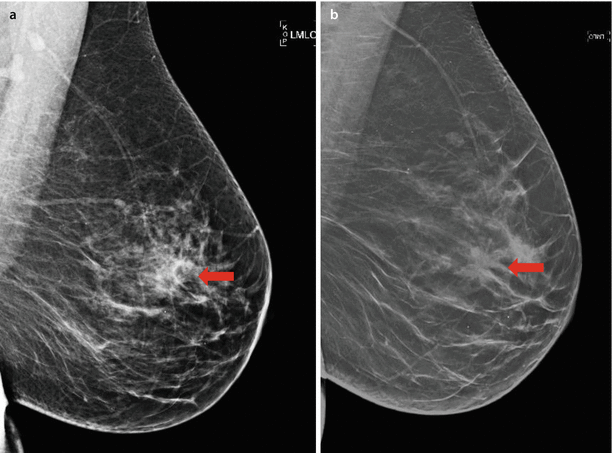

Fig. 12.8
Tomosynthesis vs. digital mammography. Carcinoma presenting as spiculated mass (arrow) is more clearly seen in tomosynthesis study b in comparison to digital mammography a
Contrast-enhanced spectral mammography (CESM) is a novel approach using a pair of low-energy and high-energy images and intravenously administered iodine-based contrast media to improve detection of pathology. Its use is currently being studied.
12.2 Breast Ultrasound
Ultrasound (or ultrasonography) is a method using high-frequency acoustic waves; the image is composed in real time based on different reflective characteristics of the tissues. For breast imaging, linear transducers with a minimum frequency of 10–12 MHz are recommended [20]. The method carries practically no risk to the tissue and can be safely used in all groups of patients, also during pregnancy and breastfeeding.
12.2.1 Indications for Breast Ultrasound
Breast ultrasound is used as a standalone diagnostic technique or in combination with other imaging modalities. Indications for breast ultrasound include:
Confirmation and characterisation of abnormalities found in other modalities
Evaluation of palpable masses and other breast-related symptoms
Axillary staging in women with breast cancer
Evaluation of breast implants
Guidance of interventional procedures in the breast and axilla
Evaluation of young, pregnant and breastfeeding patients with clinical symptoms [23]
12.2.2 Ultrasound Examination of the Breast
Ultrasound examination is performed in the supine position with the arms elevated to permit examination of all parts of the breast. Ultrasound gel enables good contact between the transducer and the skin surface. Evaluation of the image is done in real time; still images are recorded for future reference for all abnormalities as well as representative documentation of normal finding.
The quality of the examination is variable and is affected by a number of factors – the technical parameters of the machine, setting of the image and the experience of the user. Visibility of the lesion on ultrasound depends on its biological nature and size. Some lesions might have a very distinctive morphology on ultrasound, such as cysts, and some lesions might have a very subtle appearance (fibroadenomas or some malignant lesions). The majority of microcalcifications are not visible on ultrasound at all (see ◘ Table 12.3).
Table 12.3
Factors improving ultrasound performance
Technical parameters of the machine, optimal setting of the image, high-frequency transducer |
Systematic examination of the breast (in preventive setting) |
Targeted examination (with correlation to mammography, MRI or clinical finding) |
Experience of the user |
While handheld ultrasound is already widely available, automated breast ultrasound (ABUS) is currently being introduced to provide less operator dependence, higher consistency and reproducibility and less physician time for image acquisition. The image is acquired by a wide field-of-view high-frequency transducer placed on the breast with minimum compression. The images are subsequently evaluated on a workstation; viewing in multiple anatomical planes is possible to detect mass lesions as well as distortions. ABUS can be valuable especially in the preventive setting, as an additional screening tool for high-density breasts, where it improves cancer detection [24].
12.2.3 Abnormal Findings on Ultrasound
Breast lesions typically appear as space occupying masses within the tissue. Lesions are assessed by their features (shape, margins, orientation, the internal echo pattern, posterior enhancement or shadowing, the presence or absence of calcification) to estimate their biological character [25, 26].
Typical Imaging Findings
Malignant lesions typically appear as hypoechoic masses with an irregular shape, and they have poorly circumscribed (spiculated or lobulated) margins, orientation non-parallel to the chest wall/skin (taller-than-wide), with posterior shadowing; see ◘ Fig. 12.9a. Malignancy can sometimes be seen as an area of decreased echogenicity possibly with posterior shadowing.
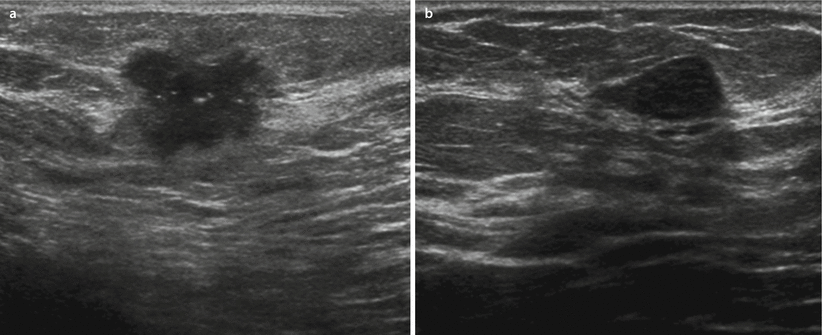

Fig. 12.9
Mass lesions in ultrasound; a malignant tumour with indistinct margins, markedly hypoechoic, with bright dots representing microcalcifications; b benign lesion – fibroadenoma – with smooth contours, parallel orientation, homogeneous structure
Benign lesions are usually oval, circumscribed, with a parallel orientation and homogeneous echo pattern with no posterior features (◘ Fig. 12.9b). If all the morphologic criteria of a benign lesion are met, the lesion does not require biopsy and undergoes follow-up within a short time interval for assurance of stability over time especially in younger women and lesions smaller than 2 cm [27]. However, whenever there is a marked increase in size or any of the descriptive characteristics is not typically benign, biopsy should be performed as some malignant tumours (especially in young women) may mimic benign lesions. These may include medullary cancers, high-grade cancers or mucinous cancers which can have appearance similar to cysts or fibroadenomas. The finding on ultrasound should be also always correlated with other imaging methods, clinical findings, age and risk factors. Attention is needed to make sure that no malignant morphological sign is missed in any of the imaging methods. In BRCA mutation carriers, any new focal lesion should be biopsied despite its benign appearance in ultrasound.
Cysts and fluid collections can be reliably differentiated from other lesions due to the typical feature of liquid content appearing black (anechoic). If in doubt, fine needle aspiration can be performed to confirm the cystic character of the lesion.
Ducts are often evaluated in patients with nipple discharge [28]. Focused ultrasound examination can sometimes reveal changes in the ducts, usually as echogenic lesions in the lumen indicating intraductal proliferation (typically papilloma), as shown in ◘ Fig. 12.10.
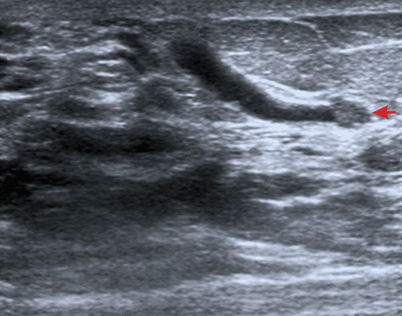

Fig. 12.10
Evaluation of a patient with nipple discharge. Dilated duct with intraluminal proliferation representing a papilloma (arrow) is clearly seen
12.2.4 Evaluation of the Axilla
Assessment of the axilla is an integral part of imaging evaluation of the breast especially when a malignancy is suspected. Ultrasound is a useful tool to predict lymph node involvement and to guide tissue sampling of the nodes [29].
To predict metastatic involvement of lymph nodes, various aspects of the node are assessed – size, shape, margins, cortical thickening, hilar compression or displacement. Normal lymph nodes typically have a thin cortex (◘ Fig. 12.11a). Metastatic involvement of the lymph node is suspected if cortical thickening (more than 2.5–3 mm) is present especially with compression or displacement of the hilum [30] (◘ Fig. 12.11b). Uniform cortical thickening may be suggestive of a range of different pathologies: reactive changes, immunological/systemic inflammatory disease or haematological malignancy such as lymphoma or chronic lymphocytic leukaemia (CLL) [31].
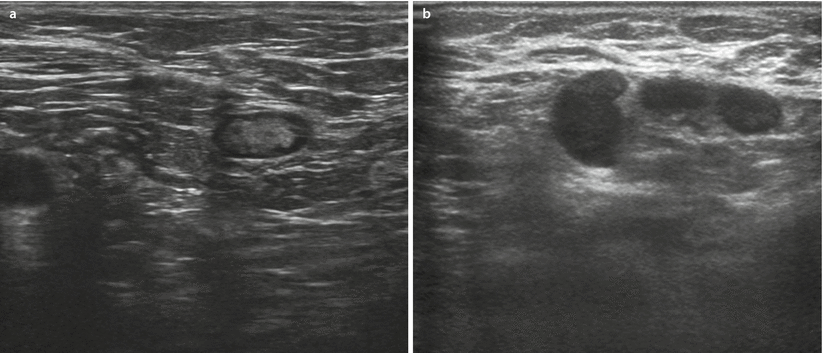

Fig. 12.11
Lymph nodes in ultrasound. Normal lymph node a and pathological b lymph nodes with marked thickening of cortex and displaced hilum
Ultrasound is also used for guidance of lymph node biopsy to confirm or rule out metastatic involvement. The diagnostic performance of ultrasound, fine needle aspiration biopsy and core biopsy of lymph nodes are shown in ◘ Table 12.4 [32, 33].
For evaluation of the axilla and nodal staging, more advanced imaging methods have been studied such as MRI (superparamagnetic iron oxide (SPIO) tracer-enhanced) or PET/CT, but their use is not yet part of routine practice.
12.3 Breast MRI
Magnetic resonance imaging of the breast (MRI, MR mammography, MRM) is increasingly used in the characterisation and diagnosis of breast pathology. The method characterises tissues based on their morphology and tissue composition which result in variable signals on different MRI sequences. Breast MRI uses application of contrast media to detect pathology and assesses the biological features of the lesions. Interpretation is complex and requires skill, experience and time.
12.3.1 Indications for Breast MRI
The indications include both screening and diagnostic use [34]:
Preoperative staging – evaluation of the extent of disease and detection of possible multifocality or multicentricity or contralateral cancer in patients with proven breast cancer.
Pretreatment evaluation of local disease extent – evaluation of the possible involvement of chest wall, vascular or nerve bundles to assess operability.
Monitoring of primary systemic therapy – MRI can evaluate early changes in the tumour caused by chemotherapy to demonstrate the effect of therapy. It is also valuable at baseline in establishing the pattern of disease in the breast and whether it is likely to undergo concentric or fragmented shrinkage and hence help to select women who may be unlikely to achieve BCS after primary systemic therapy [38].
Assessment of residual disease after primary systemic treatment [39].
Unclear findings from conventional methods – equivocal findings on mammography or ultrasound, discordance with clinical findings or differentiation of scar from recurrence; MRI is recommended only in cases when biopsy is not possible.
Detection of an occult primary tumour in patients with metastatic involvement of axillary lymph nodes (of mammary subtype on pathology) and negative mammography and ultrasound.
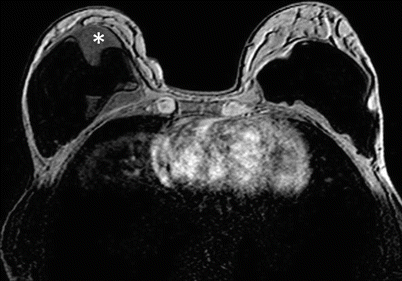
Assessment of breast implants – a non-contrast protocol with sequences specific to silicone and fluid is used (◘ Fig. 12.12).

Fig. 12.12
Evaluation of implant by MRI. Sequence with silicon suppression. The patient’s left implant (seen right on the image) is normal, while right implant is ruptured. Pathological content of mixed signal between capsules of the implant (asterisk) can be seen indicating leak of silicone, and the inner capsule is markedly folded
12.3.2 Technical Considerations and Protocol
For breast imaging, a minimum 1.5 Tesla device is used with a dedicated breast coil providing sufficient quality images with high spatial resolution. During the examination, the patient lies prone on the MRI table with her breasts loosely placed inside the coil. Gadolinium-based contrast media at a dose of 0.1 mmol/kg body weight is given intravenously by an automatic injection device to ensure constant flow.
Contraindications to MRI examination include implanted pacemakers (with the exception of MRI-compatible devices), implanted metallic material (e.g. cochlear implants, vascular stents and clips) or metallic foreign bodies, in any anatomic location. Claustrophobia may be a limiting factor. Allergies to MRI contrast media and impaired renal function must be noted [40].
Although MRI as a method is considered safe in pregnancy, its use for breast cancer detection requires administration of gadolinium contrast media which crosses the placental barrier. The gadolinium-based contrast media seems not to have teratogenic or mutagenic effects, but long-term adverse effects on the foetus and child development are possible and are still being studied, and therefore gadolinium administration in pregnancy should not be considered risk-free, and the risk-benefit ratio should always be carefully evaluated [41]. The value of MRI during breastfeeding may be limited due to the physiological changes in the lactating breast tissue, but can still provide important information. Gadolinium contrast media can be used safely as it is excreted to the breast milk; however, the amount received by the child is very low. All of the gadolinium administered to the mother is eliminated within 24 hours so if preferred breastfeeding can simply be interrupted for a day.
Examination protocols may vary between institutions. Scanning time using the full protocol ranges between 15 and 30 minutes and usually includes:
T2-weighted images (T2WI) with/without fat suppression.
T1-weighted images (T1WI) without contrast.
Dynamic T1WI after contrast media administration – series of scans with duration of 40–60s, 5–8 repetitions, images with/without fat suppression. Image subtraction supports visualisation of areas of contrast uptake.
Diffusion-weighted sequence (DWI) or MR spectroscopy (MRS) may be added to the protocol.
Orientation of the scans is variable; the typical orientations are axial (horizontal), coronal or sagittal slices, which can be used to provide better anatomical orientation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







