10 Imaged-Guided Surgery: Frame and Frameless
The development of image-guided techniques over the last 25 years has seen the progression from computed tomography (CT) frame-based stereotactic biopsy, to frameless imageguided surgical systems, to intraoperative real-time magnetic resonance imaging (MRI).
 Stereotactic Brain Lesion Biopsy
Stereotactic Brain Lesion Biopsy
Indications
Even with advances in modern brain imaging, optimal treatment still requires tissue diagnosis prior to recommending therapy in many cases. In certain situations one may entertain the possibility of forgoing biopsy particularly for the very elderly or medically infirm and initiate treatment based on imaging diagnosis. Other example situations include radiosurgery for vestibular schwannoma and metastases based on the fact that the differential diagnosis of such lesions is so narrow.
However, it is important to recognize that there is a substantial rate of unsuspected clinically significant diagnoses to be made when lesions are biopsied. This may be as high as 12 to 26% in some series, and includes pathologies such as multicentric glioma, infarct, infection, inflammation, benign cyst, and demyelination.1,2 However, modern MRI techniques have now eliminated many of these surprising pathologies with the use of magnetic resonance spectroscopy (MRS), diffusionweighted imaging, and regional or localized blood volume mapping.3–5 Today, the biopsy of basal ganglia infarcts, demyelinating periventricular plaques, and encephalitis should be an extremely rare event.
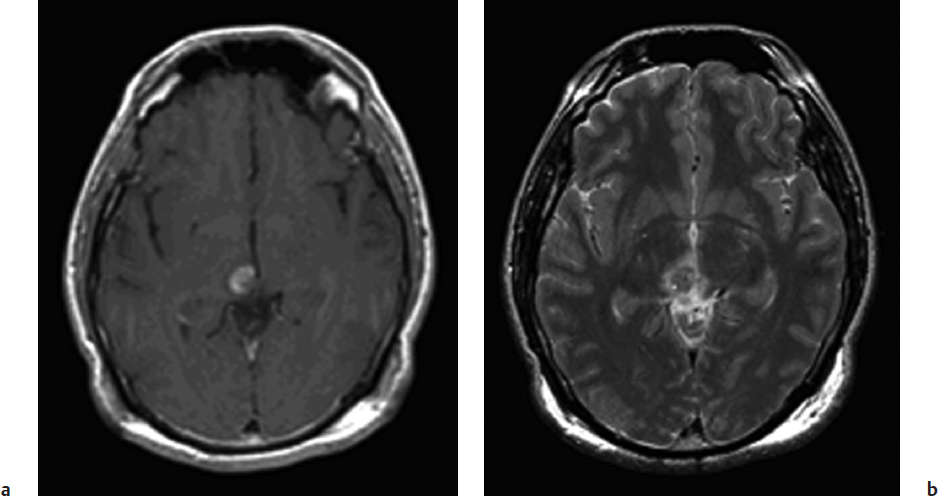
The ideal lesion for stereotactic biopsy as opposed to craniotomy is a small, deep, contrast-enhancing lesion or one located in eloquent cortex for which the risk of craniotomy and excisional biopsy is felt to be too high (Fig. 10.1).
Special Consideration
• It is good practice for a surgeon to review the diagnostic imaging with a neuroradiologist to confirm clinical impressions before proceeding with a surgical procedure.
Considerations for Stereotactic Biopsy
• Lesion
 Deep
Deep
 Small
Small
 Eloquent location
Eloquent location
 Multiple lesions
Multiple lesions
 Diffuse
Diffuse
• Patient
 Too ill
Too ill
 Too old
Too old
 Too well
Too well
 Patient preference
Patient preference
Multifocal lesions, lesions that do not fit the clinical picture, and lesions that are diffuse are also suited to stereotactic biopsy. Contrast-enhancing lesions after intense focal therapies may create a diagnostic dilemma. Whether the image abnormality represents a treatment effect or recurrent tumor, stereotactic biopsy may be necessary to confirm or refute recurrent tumor and help direct subsequent therapies.
Although many clinicians would regard eloquent brain location such as primary motor cortex as a good indication for stereotactic biopsy, a surgeon should proceed in these situations with caution. Frequently, patients with mild neurologic deficit with lesions in eloquent cortex may experience a significant transient worsening of their neurologic condition even with small-core biopsies in the absence of a documented postoperative hemorrhage. For lesions in motor speech and primary motor cortex, open awake craniotomy with functional localization of tissue before cortical incision and biopsy remains an excellent treatment option.6
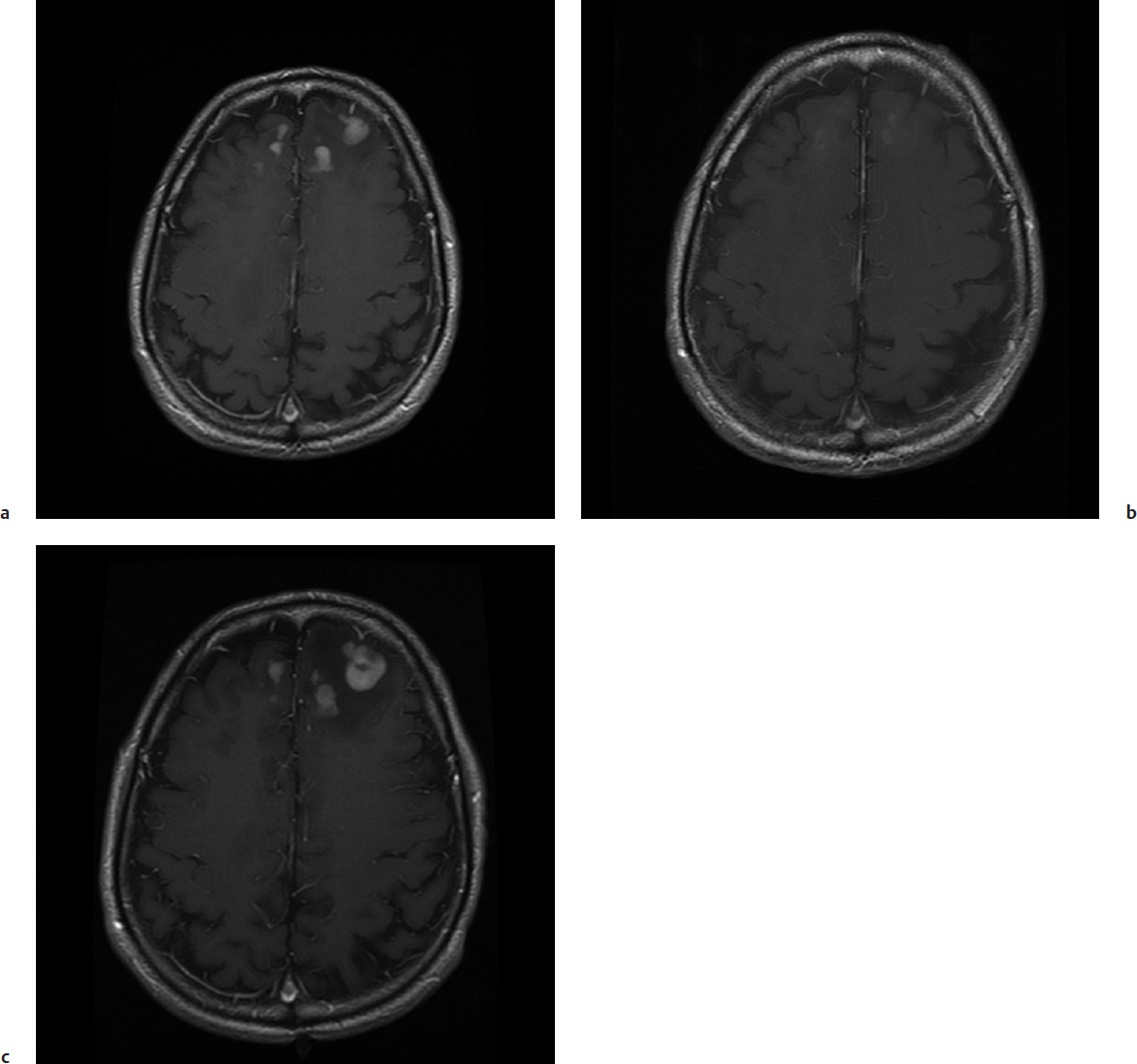
In the late 1980s and early 1990s, stereotactic biopsy had a significant role to play in the management of patients with human immunodeficiency virus (HIV) infection and AIDS.7 Subsequently, patients with multiple contrast-enhancing lesions in the setting of HIV infection were prescribed empiric antitoxoplasmosis therapy. Now this central nervous system (CNS) infection is a rare event, and stereotactic biopsy may be more important than empiric therapy. Lymphoma, bacterial infections, and progressive multifocal leukoencephalopathy remain other diagnostic considerations in this population. Care must be taken when previously obvious contrast-enhancing lesions are less intense after short courses of steroid medications, which may represent the early response of a lymphoma to steroids. Stereotactic biopsy in this setting is frequently nondiagnostic and reveals only the ghosts of cell membranes, requiring that steroid therapy be stopped and the patient observed until the lesions reappear and biopsy can be reconsidered (Fig. 10.2). One must keep in mind the high rate of complications in performing stereotactic biopsies in the HIV population, presumably related to their viral angiopathy.
Pearl
• After prior focal radiotherapy, biopsy may be necessary before proceeding with additional treatment to distinguish recurrent tumor from radiation necrosis.
Pitfall
• Dramatic reduction in the size of a contrast-enhancing lesion after steroids should raise consideration of the diagnosis of lymphoma. Biopsy in such settings will frequently result in nondiagnostic tissue.
General Technique of Stereotactic Biopsy
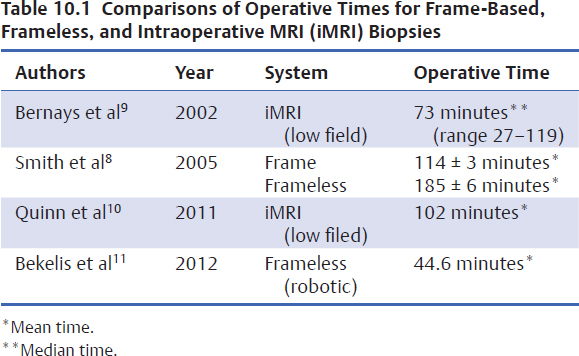
Stereotactic biopsy can currently be performed using framebased technologies, image-guided surgical systems, or intra-operative MRI, and it is effectively done under local anesthesia with intravenous sedation. Operative times vary according to the technology used (Table 10.1).8–11 The selection of anesthetic technique may affect the time to discharge and the health care costs associated with the procedure.
For frame-based procedures, the surgeon applies the frame with local anesthesia using four pins on posts. If there is a prominent skull defect, three-point fixation may be used. Positioning of the frame may be done with Velcro straps, manual handheld positioning, or the supplied ear bars, depending on the frame-based system to be used. The skin may be marked along the trajectory of the fixation pins, the frame temporarily removed, and then the scalp pin sites injected with a combination of Xylocaine (AstraZeneca, Westborough, MA) with epinephrine. Usually 1 to 2 cc at each pin site is adequate. The pins are then tightened manually using the thumb and index finger to the point where they cannot be tightened any further, and then a wrench is used. A torque wrench may be used to avoid overtightening and bending the frame.
Following frame application, the patient is taken to the imaging department where either CT or, more commonly now, MRI is done. Fiducial as well as target coordinates are then derived in the same axial imaging plane, and an entry point can be selected on coronal reconstructions to avoid structures such as the ventricle. Most biopsy needles have a side port opening of 9 to 10 mm in length and 1.2 mm in diameter. The target should be selected by placing the center of the biopsy needle port opening as close to the center of the target as possible or in the maximally enhancing area. For smaller diameter lesions, the tip of the biopsy needle should not extend past the deepest part of the lesion. If the first sample is nondiagnostic on frozen section, the depth can be advanced or shortened 2 to 3 mm in a sequential manner with the same trajectory.
In the operating room the surgeon uses either a twist drill or bur hole depending on whether the entry point is in hairbearing or non–hair-bearing scalp and on personal preference. If a twist drill hole is performed, the dura can be opened by overdrilling by a few millimeters or with successively larger sizes of Kirschner wires (K-wires), so that the stereotactic biopsy needle can be passed through the dura without difficulty. In both twist drill and bur hole situations, the standard brain biopsy needle with the side-cutting port is advanced to the target position. The needle is advanced with the side-cutting port closed and then opened at the target position to apply gentle suction, drawing tissue into the opening. While still applying gentle suction, the needle opening is closed by rotating the inner cannula 180 degrees. The entire needle, inner and outer cannula included, can then be gently rotated 360 degrees at the target position to ensure there is no resistance. If resistance is encountered, the needle may be opened briefly and then closed and rotated again to ensure it is free before removal. Surgeon preference will dictate whether the inner cannula only is removed or whether the entire biopsy needle is removed for each sample obtained. Intraoperative frozen section examination of tissue is performed to ensure that sufficient pathological tissue has been obtained. The risk of hemorrhage rises with each biopsy sample taken.
Postoperative Management
Postoperatively, patients are monitored in a recovery room setting for a period of time until they return to their base neurologic status. In one study the clinical and economic consequences of early discharge of patients following supratentorial stereotactic brain biopsy was evaluated.12 A total of 130 biopsies were performed and patient discharge status was defined as early discharge (< 8 hours) extended outpatient observation (≥ 8 eight hours but < 24 hours), and in-patient hospitalization (≥ 24 hours). All complications, of which five were serious, occurred within 6 hours after surgery. Intraoperative bleeding occurred in 12 patients (9.2%), but in only 40% of these cases did hemorrhage appear on postoperative CT scans. The authors concluded that early discharge of patients following stereotactic biopsy was safe in the absence of excessive intraoperative bleeding, development of new postoperative deficits, or demonstrated blood clot formation on a postoperative CT scan.
One group has prospectively assessed the feasibility of outpatient surgery, that is, discharge home 4 to 6 hours after stereotactic biopsy.13,14 The authors started doing stereotactic biopsy on an outpatient basis in 1997, and published their pilot data in 2002 and an update in 2011. A total of 152 patients were in the intent-to-treat group and 94.1% were successfully discharged home without readmission. In the 5.9% protocol failure group two neurologic complications occurred (2.6%), and neither of these patients had hemorrhage on postbiopsy CT scans. The authors concluded that discharging patients home after 4 to 6 hours of observation and CT following stereotactic biopsy seemed to be safe and was expected to result in a savings of health care resources and costs, with a potential improvement in patient satisfaction and decreased complications because of the shorter hospital stay.
Controversy
• Stereotactic tumor biopsy can safely be done on an outpatient basis.
Complications
Although it is easy for neurosurgeons, and even for other physicians, to think of stereotactic biopsy as a simple minimally invasive procedure, one should resist this temptation. A thorough knowledge of the anatomy and of the trajectories to the target is necessary to avoid both intraoperative patient discomfort and postoperative disability. Should complications arise in the postoperative period, early recognition and treatment are necessary to maintain good patient outcomes. A comprehensive knowledge of these complications is essential to allow for their proper avoidance as well as informed discussion with the patient prior to the procedure.
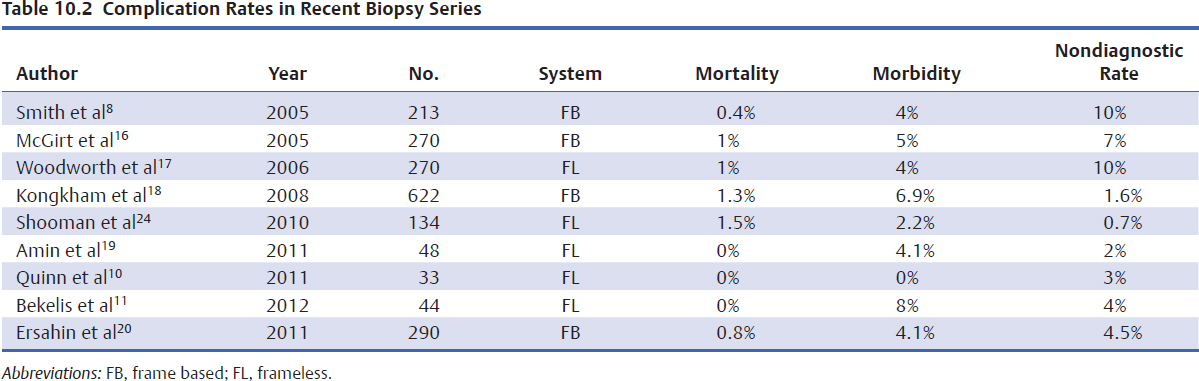
Hemorrhage at the biopsy site, causing acute deterioration and new neurologic deficits, is the most feared complication of stereotactic brain biopsy. The incidence of neurologic morbidity and mortality, mostly due to hemorrhage, ranges from 0 to 5% in larger series (Table 10.2).11–20 Clinically silent hemorrhage after stereotactic biopsy is very common. On CT scans obtained in 102 patients who underwent stereotactic biopsy, 59% of the scans exhibited hemorrhages.21 In only six of the 61 patients were there clinical signs to suggest the patient may have suffered a hemorrhage, whereas in the remaining 55 the hemorrhage was clinically silent and unsuspected. The clinically silent hemorrhages ranged in size from less than 5 mm to between 30 and 40 mm. Of the 55 patients with clinically silent hemorrhages, only three demonstrated a new neurologic deficit, and all these occurred within 2 days of the procedure. All the patients with no hemorrhage on postoperative scans remained neurologically well and experienced no delayed deterioration. In a more recent series, hematoma at the biopsy site was seen in only 9% of patients, of whom four were symptomatic.16 High-grade glioma was a risk factor for hematoma at the biopsy site but was not associated with a significant increase in morbidity.
All necessary steps should be taken preoperatively to help avoid hemorrhage, beginning with proper patient screening and obtaining a full hematological and coagulation profile when indicated. Patients are counseled to avoid nonsteroidal antiinflammatories for 10 days prior to the planned procedure. Care should be taken to avoid targeting lesions in areas close to large arteries or veins such as the pineal region or perisylvian region, and superficial lesions near the pia-arachnoid. Avoidance of intraoperative complications calls for careful surgical technique while passing the needle to the biopsy site and taking the minimum number of samples necessary to satisfactorily obtain diagnostic tissue. If bleeding is encountered after the first biopsy, the first step is to leave the biopsy instrument in place with the side port open so that blood may exit through the needle. In patients who deteriorate neurologically in the operating room, the surgical team must be prepared to remove the stereotactic head frame rapidly should endotracheal intubation be necessary.
Special Consideration
• If hemorrhage occurs during biopsy, it is best to leave the needle in place with the side port open so that blood can escape through the needle.
Postoperative management of suspected intraoperative bleeding involves close monitoring of the patient and then an early CT scan. If the patient’s condition deteriorates quickly, emergency craniotomy may be required for more superficial lesions, whereas deep basal ganglia lesions may be managed with medical measures. In some cases, endoscopic or repeat stereotactic aspiration of hematomas may be of benefit. In certain cases in which the biopsy has shown a malignant neoplasm in an elderly patient who has developed a severe neurologic deficit due to a biopsy-induced hemorrhage, it may be appropriate after a thorough discussion with the patient’s family to forgo craniotomy and provide only comfort measures. It is important to have such discussions with the patient and family prior to the procedure to determine their wishes.
Failed (Nondiagnostic) Biopsy
In most large clinical series, stereotactic biopsy results in nondiagnostic tissue sampling in less than 10% of cases.17,22 Nondiagnostic tissue samples may be associated with nonneoplastic or nonenhancing lesions. Patients on prior steroid medications whose lesions are small at the time of stereotactic imaging may have lymphoma, which is extremely sensitive to steroid medications. Biopsy in this setting usually results in only necrotic tissue being obtained. Failed biopsies are more frequently associated with increased complications and failure to conduct an intraoperative pathological examination.
Pitfall
• Failure to use intraoperative frozen section will result in a higher rate of nondiagnostic biopsy.
The management of the failed biopsy begins in the operating room, where intraoperative examination of tissues is mandatory to ensure that diagnostic tissue is in fact obtained. Repeated sampling may increase the diagnostic yield, and the window of the side-cut needle can be changed in its orientation among 0, 90, 180, and 270 degrees for each sample. As well, the depth of the sampling point within the target lesion can be modified. If diagnostic tissue is not obtained, all parameters and measurements must be rechecked to ensure accurate localization, and then a repeat biopsy sample can be sent. If repeated samples do not provide diagnostic tissue, then the surgeon must weigh the risk of further attempts. Sending tissue for microbiological analysis as well as pathology must be considered for lesions that may be inflammatory or infectious.
Postoperatively an immediate CT scan can help localize the biopsy site, usually marked by a drop of air or blood to ensure that the appropriate location was sampled. With the use of image-guided systems, screen saves of the needle at the target position can be used as a record of correct target localization. If a final pathological examination is still not diagnostic, then the options include repeat biopsy, open biopsy, or empiric therapy.
Sampling Error
Stereotactic biopsy specimens provide tissues representative of only a small portion of the lesion. Frequently the target selected is based on static postcontrast MRI or CT, which may not be the best predictor of the most metabolically active component of a tumor. It is known that malignant gliomas have a great deal of heterogeneity within their volume, and surgeons must intentionally avoid areas of suspected nonenhancing necrotic tissue in selecting their target and avoiding cystic areas. In the latter case, draining the cyst first may change the morphology of the lesion before tissue is obtained, altering the intended target for sampling. With modern magnetic resonance (MR) techniques, MRS can help localize the area of greatest abnormality; MR perfusion techniques may have similar value in this regard.3,5,23 In 29 patients with brain tumors who underwent high-resolution three-dimensional (3D) MRS before undergoing surgery, biopsies obtained at locations referenced on MRI were compared with MR spectral voxels centered on each of 79 biopsy locations, and metabolic levels were correlated with histological examinations of each specimen.4 Elevated normalized choline ratios and low normalized N-acetylaspartase ratios were associated with viable tumor 90% of the time. Such spectroscopic information can be used particularly for low-grade, nonenhancing lesions to more accurately direct the biopsy target to the most abnormal area with elevated choline levels.
• Metabolic imaging studies may be useful for directing biopsy of larger nonenhancing tumors or lesions where diagnosis based on postcontrast imaging is in question.
The question of whether intraoperative frozen section analysis should routinely be done has been addressed. Using one to three specimens from a single trajectory, 99.3% of biopsies done using a frameless system were diagnostic.24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree