Fig. 4.1
T1-weighted coronal and sagittal MR images show diffusive enlargement and enhancement of the pituitary gland. The gland pyramidal in shape extending to hypophyseal stalk from the sellar base. Images also note the presence of enhanced mucosa in the bilateral sphenoid sinus and enhanced meninges around the sella. Images: preoperative (a), postoperative (b), 6 months post-high-dose GC treatment (c), 3-year follow-up off high-dose GC (d)
On review of symptoms she reported fatigue, nausea, which improved after starting steroids, brittle fingernails, edema in the legs, weakness in all extremities, memory deficits, sleep disturbances, and a history of chronic bronchitis with persistent shortness of breath.
Her family history was significant for heart disease in her mother, brother, aunt, uncles, and grandfather and tuberculosis in a great aunt.
In addition to treatment with prednisone daily at 5 mg (she was subsequently switched to hydrocortisone (HC) at a dose of 20 mg daily), medications included Azmacort, Nasonex, albuterol, a statin plus Zetia and niacin, Vivelle-Dot estrogen replacement, calcium, and multivitamins.
On physical exam she was normotensive, blood pressure was 136/69 mmHg, pulse 80, respirations 16, weight 156 lbs, and height 5ʹ8ʺ. Head, ears, eyes, nose, and throat (HEENT) exam revealed a temporal visual field (VF) deficit in the right eye and a formal VF exam indicated right temporal field restriction (Fig. 4.2). The remainder of her HEENT exam was unremarkable with extraocular movements and cranial nerves grossly intact. Pituitary function testing was performed including cortrosyn stimulation testing for adrenal insufficiency (AI); after holding HC for 24 h, AI was confirmed (Table 4.1). A diagnosis of central hypothyroidism with low free T4 and inappropriately low TSH was evident and treatment with levothyroxine was initiated. Growth hormone (GH) deficiency was suspected based on low insulin growth factor-1 (IGF-1); prolactin (PRL) was low. Based on a presumed diagnosis of nonfunctioning pituitary adenoma, the patient underwent transsphenoidal surgery (TSS) for optic chiasm decompression. In retrospect, a few MR imaging features were possibly suggestive of hypophysitis such as mild stalk thickening, sellar mass with cystic areas, and a vague ring enhancement; these are not very specific and her atypical clinical presentation in addition to VF loss warranted optic chiasmal decompression and at least a biopsy for final diagnosis. Classic features of MR imaging in hypophysitis include the presence of a “dural tail” (enhanced tissue along the dura mater), the absence of mucosal thickening in the sphenoid sinus, isointensity with gray matter on T1-weighted images, and marked homogeneous or heterogeneous enhancement by gadolinium (Table 4.2).

Fig. 4.2
Visual field exam indicating right temporal field restriction
Table 4.1
Results from laboratory test analysis
Laboratory test (unit) | Reference range | Preoperative | 4 weeks postoperative |
|---|---|---|---|
Glucose (mg/dL) | 65–110 | 115 | 52 |
BUN (mg/dL) | 6–20 | 13 | 16 |
Creatine (plasma mg/dL) | 0.6–1.1 | 0.9 | 1.2 |
Total protein (g/dL) | 6.1–7.9 | ||
Albumin (g/dL) | 3.5–4.7 | 4 | |
Calcium (mg/dL) | 8.5–10.5 | 9.7 | 9.8 |
Sodium (mmol/L) | 136–145 | 140 | 145 |
Potassium (mmol/L) | 3.5–5.1 | 3.7 | 4.1 |
Chloride (mmol/L) | 98–107 | 104 | 103 |
Total CO2 (mmol/L) | 23–29 | 23 | 29 |
Alanine aminotransferase (U/L) | 13–48 | 64 | |
Bilirubin (direct mg/dL) | Low: <0.4 | ||
Adrenocorticotropic hormone (plasma pg/mL) | Low: <46 | <5 (L) | <5 (L) |
Serum cortisol (total μg/dL) baseline | 5.0–23.0 μg/dL | <1 (L) | 1.2 |
Serum cortisol (total μg/dL) post-stimulation | >18 μg/dL | 2.1 (L) | 3.0 |
Prolactin (ng/mL) | 3–29 ng/mL | <1 | <1 |
Lutenizing hormone (mIU/mL) | <1 | ||
Thyroid stimulating hormone (μIU/mL) | 0.28–5.00 | 0.08 (L) | 0.010 (L) |
Free T4 (ng/dL) | 0.7–1.8 | 0.4 (L) | 1.7 on replacement |
Follicle stimulating hormone (mIU/mL) | <1 | ||
IGF-1 | 64–188 ng/mL | 66 | 27 |
Table 4.2
Imaging technique findings: lymphocytic hypophysitis vs. pituitary adenoma
Imaging technique | Finding | Lymphocytic hypophysitis | Pituitary adenoma |
|---|---|---|---|
Sellar X-ray | Sellar floor | – Intact – Uniformly flat | – Not intact – Unilateral depression |
Pituitary MR | Pituitary | – Enlargement – Symmetrical sovrasellar expansion | – Unilateral endosellar mass (microadenoma) or inhomogeneously expanding mass – Asymmetrical sovrasellar extension |
Chiasm | – Compression – Displacement | – No compression (microadenoma) – No displacement (microadenoma) | |
Stalk | – Thickened – Not deviated | – Not thickened – Contralateral deviation | |
After gadolinium | Pituitary mass enhancement | – Intense – Homogeneous | – Slight, delayed – Inhomogeneous (focal) |
“Dural tail” | – Appearance | – Lacking | |
“Bright spot” of neurohypophysis | – Loss (if DI is associated) | – Persistence |
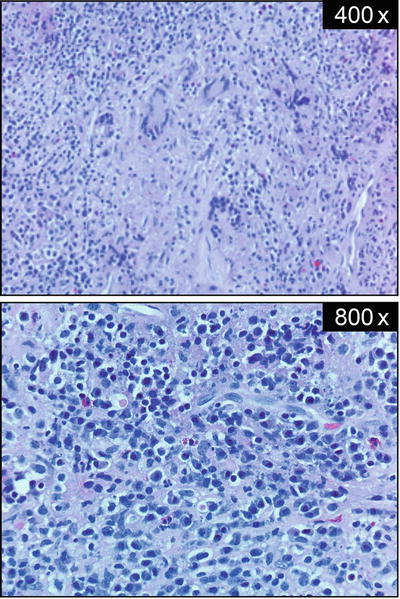
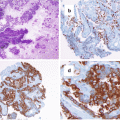
How Was the Diagnosis of Granulomatous Hypophysitis Made?
Pathology revealed necrotizing granulomas with giant cells and marked lymphoplasmacytic infiltration (Fig. 4.3). Rare acini of anterior pituitary parenchyma were also identified. Stains for microorganisms, bacilli, and fungi (Steiner, acid-fast bacilli, and Gomori methenamine silver) were negative and CD1a staining was also negative (Fig. 4.4). Reticulin staining revealed intact acinar structures, synaptophysin highlighted the native gland, and human GH was focally positive. Staining for PRL and adrenocorticotropic hormone (ACTH) was negative. There was no evidence of a tumor and no vasculitis was present in the specimen. In view of these findings, a diagnosis of granulomatous hypophysitis (GrH) with a component of lymphocytic hypophysitis (LH) was made.